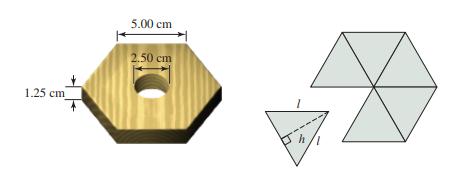
Consider a 58.35 g hexagonal block of wood that is 5.00 cm on edge and 1.25 cm
Question:
Consider a 58.35 g hexagonal block of wood that is 5.00 cm on edge and 1.25 cm thick, with a 2.50 cm diameter hole drilled through its center. Also given are the densities of the liquids hexane (d = 0.667 g/m/L) and decane (d = 0.845 g/mL). Assume that the density of a mixture of the two liquids is a linear function of the volume percent composition of the solution. Determine the volume percent of hexane required in the solution so that the hexagonal block of wood will just barely float on the solution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





