Consider the molecule with the Lewis structure given below. (a) How many and bonds are
Question:
Consider the molecule with the Lewis structure given below.
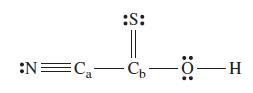
(a) How many σ and π bonds are there?
(b) What is the appropriate hybridization scheme for each of Ca, Cb, and O?
(c) In which orbitals are the lone pairs located?
(d) What are the (ideal) values of the following bond angles?
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





