For each of the following ions, write two equationsone showing its ionization as an acid and the
Question:
For each of the following ions, write two equations—one showing its ionization as an acid and the other as a base:
(a) HSO3-;
(b) HS-;
(c) HPO4-.
Then use data from Table 16.5 to predict whether each ion makes the solution acidic or basic.
Table 16.5
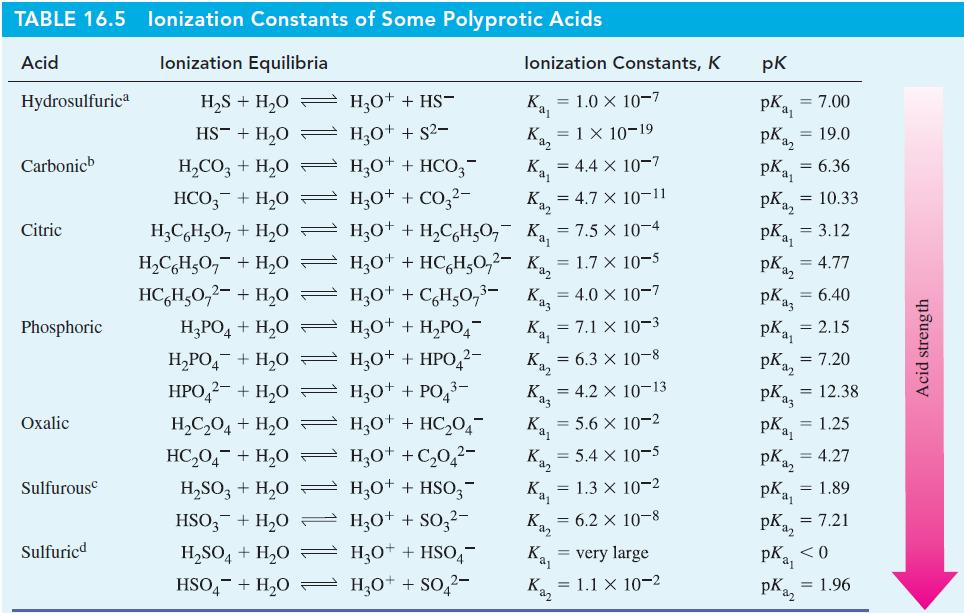
Transcribed Image Text:
TABLE 16.5 lonization Constants of Some Polyprotic Acids Acid lonization Equilibria Hydrosulfurica Carbonicb Citric Phosphoric Oxalic Sulfurousc Sulfuricd H₂S + H₂O HS + H₂O H₂CO3 + H₂O HCO3 + H₂0 H₂PO4+H₂O H₂PO4¯ + H₂0 — HPO42- + H₂O → H₂C₂O4 + H₂0 HC,O4 + H,O H₂SO3 + H₂O — HSO3 + H₂0 H₂O+ + HS¯ H₂O+ + S²- — H₂0+ + HCO3- H3O+ + CO3²- Kaj K₁₂ = 4.7 x 10-11 H₂CH₂O + H₂O H₂O+ + H₂CH₂O₂K₁₁ = 7.5 × 10-4 H₂CH₂O₂ + H₂O H₂O+ + HC6H₂0,²- K₁₂ = 1.7 x 10-5 HC₂H₂0₂² + H₂0 — H3O+ + C¿H²0,³- Kaz = 4.0 x 10-7 K₁₁ = 7.1 × 10-3 K₁₂ = 6.3 × 10-8 = 4.2 X 10-13 Kaz Ka₁ = 5.6 x 10-2 Kaz H₂O+ + H₂PO4¯ H3O+ + HPO ²− H₂0+ + PO4³- H30+ + HC₂04- H₂O+ + C₂0₂²- H3O+ + HSO3- H3O+ + SO3²- lonization Constants, K = 1.0 × 10-7 H₂SO4+H₂O → H3O+ + HSO4- HSO4+H₂O H₂O+ + SO4²- Kaj Kaz Kay Kaz = 1 × 10-19 Kaz = 4.4 x 10-7 = 5.4 x 10-5 = 1.3 x 10-2 = 6.2 × 10-8 Kaj very large = 1.1 x 10-2 pk pka₁ = 7.00 pka2 pkal pka2 = 10.33 PK₁₁ = 3.12 PK₁₂ = 4.77 = 19.0 = 6.36 pK₁3 = 6.40 pK₁₁ = 2.15 PK₁₂ = 7.20 pk az pka₁ pKaz = 12.38 pK₁₁ = 1.25 pka₂ = 4.27 pka₁ = 1.89 pka₂ = 7.21 <0 = 1.96 Acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a HSO3 Acid ionization HSO3 H2O H3O SO3 Base ionization HSO3 H2O HS OH According to T...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
For each of the following brief scenarios, assume that you are reporting on a clients financial statements. Reply as to the type(s) of opinion (per below) possible for the scenario. In addition: ...
-
If the appropriate discount rate for the following cash flows is 7.13 percent per year, what is the present value of the cash flows? Year Cash Flow 1 ......................$1,400 2...
-
Find the conversion (or stock) value for each of the $1,000-par-value convertible bonds described in the following table. Current market price of stock $42.25 50.00 44.00 19.50 Conversion Convertible...
-
Andy Confer, production-line manager, had arranged a visit with Will Keating, plant manager. He had some questions about the new operational measures that were being used. Andy: Will, my questions...
-
Consider the net cash flows and salvage values shown below. Assume the alternatives can be indefinitely renewed with the same cash flows and salvage values. Using a MARR of 8%, specify the planning...
-
Beverly Crusher, a new staff accountant, is confused because of the complexities involving accounting standard-setting. Specifically, she is confused by the number of bodies issuing financial...
-
Use conversion algorithm to convert ER diagram to Relational Model Or All ER to relational model intermediatory conversion steps with final relational model (i.e. step 4) RUberID REmail Passwd...
-
What is the pH of an aqueous solution that is 0.123 M NH 4 Cl?
-
What is the pH of an aqueous solution that is 0.089 M NaOCl?
-
Can a steady state device have boundary work?
-
Clarence Darrow, counsel for Leopold and Loeb, once said 'Justice has nothing to do with what goes on in a courtroom; Justice is what comes out of a courtroom". This week you have read about...
-
Please describe the Agreement on Mutual Recognition between the European Community and the United States with respect to pharmaceutical products. Please be sure to include the main points of the...
-
What do you think about it? Under what circumstances does utilizing a fingerprint to access an electronic device a violate the Fifth Amendment? The Fifth Amendment provides that no person "shall be...
-
Your assignment: Take a time machine back to the point at which the three partners are planning on opening the restaurant. At this point you do not know that A.J. will be a disaster or that the place...
-
Find the derivative of each function. Use the power, product, quotient, chain, exponential, and logarithmic rules as needed a) f'(x) if f(x) = x* -In(x + x) (5r-2)* b) y' if y=
-
The J. S. Bach Foundation is a non-profit charitable institution dedicated to providing musical education to children in elementary schools. There is a provision in the document that created the...
-
Four GWU students have been selected to taste food sold by 3 different food trucks labeled as food truck A, B and C on H & 22nd Streets every Monday for 3-weeks. For each student, food trucks are...
-
Chalet Sports sells hunting and fishing equipment and provides guided hunting and fishing trips. Chalet Sports is owned and operated by Cliff Owen, a well-known sports enthusiast and hunter. Cliffs...
-
The total assets and total liabilities of Coca-Cola and PepsiCo are shown below. Determine the owners' equity of each company. Coca-Cola (in millions) Pepsico (in millions) Assets Liabilities $29,963...
-
The total assets and total liabilities of eBay and Google are shown below. Determine the owners equity of eachcompany. Google (in millions) eBay (in millions) $18,473 1,433 Assets Liabilities $13,494...
-
A company set its predetermined overhead rate at the beginning of the year using the following estimates: overhead costs, $2,243,780, and direct labor costs, $434,000. Actual costs for the year are...
-
Evander bought a house on June 1, 2021. The previous owners had been foreclosed on, and Evander was able to buy the house below market, for $155,000. He made no improvements while he owned it. On...
-
Justify Australia s approach of imposing its set of accounting standards on all reporting entities, irrespective of whether they are profit seeking.

Study smarter with the SolutionInn App


