For the first-order reaction t 1/2 = 22.5 h at 20 C and 1.5 h at 40
Question:
For the first-order reaction
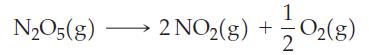
t1/2 = 22.5 h at 20 °C and 1.5 h at 40 °C.
(a) Calculate the activation energy of this reaction.
(b) If the Arrhenius constant A = 2.05 x 1013 s-1, determine the value of k at 30 °C.
Transcribed Image Text:
N₂O5 (g) 2 NO₂(g) + O2(8) 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Part a Calculate the activation energy of this reaction The Arrhenius equation relates the rate cons...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A person walks from point A to point B as shown in Fig. 3.31. What is the persons displacement relative to point A? 40 m 20 m 30 m 20 m 30
-
Kurt, an accountant at Mercury Industries, is reviewing his companys financials. He has gathered the following information: The companys Assets total $20 million. The company has debt of $12 million....
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Patients who undergo chronic hemodialysis often experience severe anxiety. Videotapes of progressive relaxation exercises were shown to one group of patients and neutral videotapes to another group....
-
Refer to Question 2. How will your answer change if the discount rate you use is increased by 0.5% (50 basis points)? Decreased by 0.5%? Comment. Both Sprint and Nextel started business on the...
-
What is the spectrochemical series? Use the ligands CN, H2O, Cl, and NH3 to illustrate the term. Then arrange them in order, describing the meaning of this order.
-
7. Assume that a subsidiary has 10,000 shares of stock outstanding, of which 8,000 shares are owned by the parent. What equity method adjustment will be necessary on the parent books if the...
-
A Pentecostal nurse claims she was constructively discharged after refusing to assist in medical procedures she considered to be abortions because of her religious beliefs. She was initially...
-
Chavez Company most recently reconciled its bank statement and book balances of cash on August 31 and it reported two checks outstanding, No. 5888 for $1,065 and No. 5893 for $519. The following...
-
A commonly stated rule of thumb is that reaction rates double for a temperature increase of about 10C. (This rule is very often wrong.) (a) What must be the approximate activation energy for this...
-
The reaction C 2 H 5 I + OH - C 2 H 5 OH + I - was studied in an ethanol (C 2 H 5 OH) solution, and the following rate constants were obtained: 15.83 C, k = 5.03 x 10 -5 ; 32.02 C, 3.68 x 10 -4 ;...
-
Anyone with a radio receiver can listen to public radio, which is funded largely by donations. a. Is public radio excludable or nonexcludable? Is it rival in consumption or nonrival? What type of...
-
Could I obtain assistance with these . problems? 1. Find the coordinates of the turning points of the curve y=3x^4-8x^3-30x^2+72x+5. Determine the nature of these points. "Determine the nature"...
-
1 . In 1 9 6 0 the homeownership rate in the United States was 6 2 % . Is there evidence to indicate that the homeownership rate is now higher? To answer the question, the researchers sample 5 0 2...
-
A certain disease is classified into 4 stages that distinguish how developed the disease is. Researchers studying a new potential treatment recruited over 100 patients with varying stages of the...
-
1. (20) Let and Dor {abnm or 2n m} = Dand = {a"b" nm and 2n m}. Prove that Dor and Dand are both context-free.
-
Given n samples 1 , 2 , . . . , x 1 ,x 2 ,...,x N drawn independently from a Poisson distribution unknown parameter , find the MLE of . = = 1 MLE = i=1 n x i = = 1 MLE =n i=1 n x i = = 1 MLE = i=1 n...
-
When designing patterns for casting, patternmakers use special rulers that automatically incorporate solid shrinkage allowances into their designs. Therefore, a 12-in. patternmaker's ruler is longer...
-
Would you use the adjacency matrix structure or the adjacency list structure in each of the following cases? Justify your choice. a. The graph has 10,000 vertices and 20,000 edges, and it is...
-
Every company needs to plan in order to move forward. Its top management must consider where it wants the company to be in three to five years. Like a company, you need to think about where you want...
-
(a) How does the time period assumption affect an accountants analysis of business transactions? (b) Explain the terms fiscal year, calendar year, and interim periods.
-
State two generally accepted accounting principles that relate to adjusting the accounts.
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


