Question: For the reaction A + 2 B C + D, the rate law is rate of reaction = k[A][B]. (a) Show that the following
For the reaction A + 2 B → C + D, the rate law is rate of reaction = k[A][B].
(a) Show that the following mechanism is consistent with the stoichiometry of the overall reaction and with the rate law.
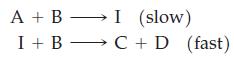
(b) Show that the following mechanism is consistent with the stoichiometry of the overall reaction, but not with the rate law.
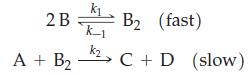
A+BI (slow) I + B C + D (fast)
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
a To show that the given mechanism is consistent with the stoichiometry of the overall reaction and with the rate law we need to ensure that the sum o... View full answer

Get step-by-step solutions from verified subject matter experts


