From the data in Table 20.2 and Figure 20-2 for the decomposition of H 2 O 2
Question:
From the data in Table 20.2 and Figure 20-2 for the decomposition of H2O2, determine (a) the initial rate of reaction, and (b) [H2O2]t at t = 100 s, assuming that the initial rate is constant for at least 100 s.
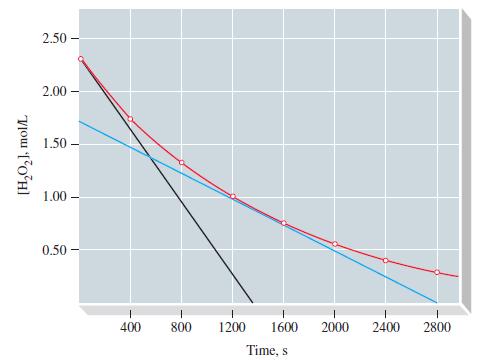
Table 20.2
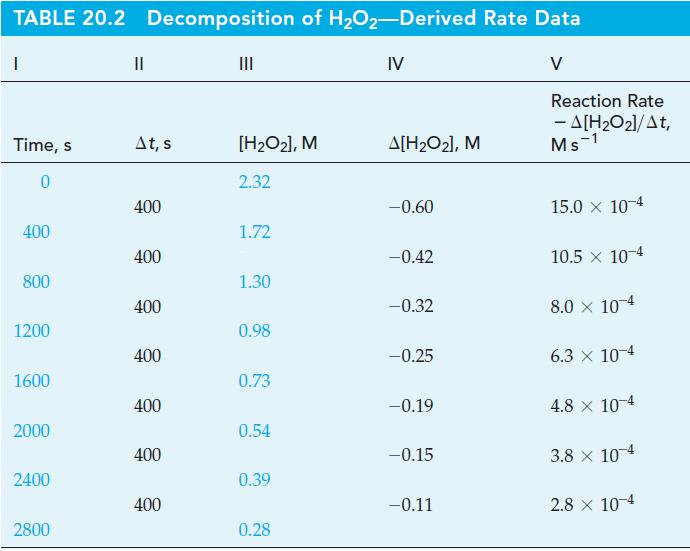
Figure 20-2
Transcribed Image Text:
TABLE 20.2 Decomposition of H₂O2-Derived Rate Data IV I Time, s 0 400 800 1200 1600 2000 2400 2800 II At, s 400 400 400 400 400 400 400 ||| [H₂O₂], M 2.32 1.72 1.30 0.98 0.73 0.54 0.39 0.28 A[H₂O₂], M -0.60 -0.42 -0.32 -0.25 -0.19 -0.15 -0.11 V Reaction Rate - A[H₂O₂]/At, Ms 1 15.0 x 10-4 10.5 x 10-4 8.0 × 10-4 6.3 x 10-4 4.8 x 10-4 3.8 x 10-4 2.8 x 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Analyze To determine the initial rate of reaction from the slope of the tangent line in part a we us...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) For reaction (20.3), determine (a) The instantaneous rate of reaction at 2400 s and (b) [H 2 O 2 ] at 2450 s. Assume that the instantaneous rate of reaction at 2400 s holds constant for the next...
-
The Tastee Bakery Company supplies a bakery product to many supermarkets in a metropolitan area. The company wishes to study the effect of the height of the shelf display employed by the supermarkets...
-
From the data in Table 14-1 in the text, calculate the overall rate change of first-class postage as measured by the LCI for the following decades: In Table 14.1 (a) The 1970s (1970-1979) (b) The...
-
Roberts Originals Co. (ROC) provides new and unique cases and otherassignments to professors each semester to ensure that students will not be able to find the solutions published online. Due to the...
-
To help you become familiar with the accounting standards, this case is designed to take you to the FASBs Web site and have you access various publications. Access the FASBs Web site at www.fasb.org....
-
1. What are the ethical issues in this case? 2. Do you think that either group, pro-GM or anti-GM foods, is correct while the other group is wrong? If so, what reasoning do you give for supporting...
-
Northern Group, Inc., is a Wisconsin corporation that contracts with manufacturers and suppliers to develop marketing strategies and promotions to third-party retailers. In exchange for these...
-
Flexible-budget preparation and analysis. Bank Management Printers, Inc., produces luxury checkbooks with three checks and stubs per page. Each checkbook is designed for an individual customer and is...
-
Explain Suppose the government grants $2,500 per child to households that have more than two children. Draw a graph and explain whether these child allowances influence the fertility behavior of...
-
The rate of decomposition of gaseous acetaldehyde, CH 3 CHO, to gaseous methane and carbon monoxide is found to increase by a factor of 2.83 when the initial concentration of acetaldehyde is doubled....
-
As shown later in the chapter, for certain reactions the initial and instantaneous rates of reaction are equal throughout the course of the reaction. What must be the shape of the concentrationtime...
-
The income statement of TorMax Technology Inc. for the year ended December 31, 2015, is as follows: Instructions Using the information from Problem 16-2B and the additional information provided,...
-
What is the time during which audience members may stand and stretch, use the restroom, or purchase refreshments?
-
Option 2 - Linked List - Josephus Problem Linked List Specification: Create a Templated/Generic Linked List. This can be a Singly Linked List, a Singly Circular Linked List, a Doubly Linked List or a...
-
Emma owns 40% of FitMe, Inc. She sells the corporation workout equipment with a fair market value of $45,000. The corporation pays Emma $55,000 for the equipment. How much income, if any, does Emma...
-
Data regarding Lager Ltd.'s production for the year is given below: Cost per unit Amount in dollars Direct labour $15.25 Direct material 9.00 Applied manufacturing overhead 6.75 Additional...
-
Student has asked to develop an enterprise application " Online Hotel Booking" to implement the CRUD with Spring Boot MVC and Spring Data JPA tools . The web application should have the following...
-
In 2011, Wade Window and Glass changed its inventory method from FIFO to LIFO. Inventory at the end of 2010 is $150,000. Describe the steps Wade Window and Glass should take to report this change.
-
Use nodal analysis to determine voltages v1, v2, and v3 in the circuit Fig. 3.76. Figure 3.76 4 S 3i, 2 A 4A
-
What new rules were enacted under the Sarbanes-Oxley Act to address unethical accounting practices?
-
Stan Kaiser is studying for his next accounting examination. Explain to Stan what he should know about the differences between the income statements for a manufacturing and for a merchandising...
-
Terry Lemay is unclear as to the difference between the balance sheets of a merchandising company and a manufacturing company. Explain the difference to Terry.
-
Selected data from the 2019 financial statements of KRJ Manufacturing are presented below. On the company's common-sized balance sheet, what would be the amount (i.e., percentage) shown for long-term...
-
Selected data from the 2019 financial statements of KRJ Manufacturing are presented below. On the company's common-sized balance sheet, what would be the amount (i.e., percentage) shown for...
-
a) Figure 11 shows a plastic component to be manufactured using injection moulding process. Sketch an appropriate parting line on the component and justify your answer. Figure 11 [Rajah 11]

Study smarter with the SolutionInn App


