From the observation that 0.0500 M vinylacetic acid has a freezing point of -0.096 C, determine K
Question:
From the observation that 0.0500 M vinylacetic acid has a freezing point of -0.096 °C, determine Ka for this acid.
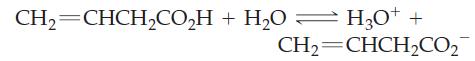
Transcribed Image Text:
CH2=CHCH,CO,H+H,O
CH2=CHCH,CO,H+H,O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the acid dissociation constant Ka of vinylacetic acid from the given information follow ...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A 0.500 m solution of MgCl2 has a freezing point of 2.60C. What is the true van't Hoff factor of this ionic compound? Why is it less than the ideal value?
-
A solution of C2H2O4 in CH3COOH has a freezing point of 10.00C. What is the molality of the solution?
-
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these...
-
Average rates of return on Treasury bills, government bonds, and common stocks, 1900-2020. Average Annual Average Premium (Extra Rate of Return return versus Treasury (8) bills) (%) Portfolio...
-
Willow Enterprises is considering the acquisition of Steadfast Corp. in a stock swap transaction. Currently, Willows stock is selling for $45 per share. Although Steadfasts shares are currently...
-
Milton Corporation manufactures skateboards and is in the process of preparing next years budget. The pro forma income statement for the current year is as follows: Required: 1. What is the...
-
A project has been selected for implementation. The net cash flow (NCF) profile associated with the project is shown below. MARR is 10 percent/year. a. What is the internal rate of return of this...
-
Knott Radio Corporation is a subsidiary of Mercer Companies. Knott makes car radios that it sells to retail outlets. It purchases speakers for the radios from outside suppliers for $28 each....
-
A combined solar and auxiliary energy system is used to meet the same load as in Example 12.5. The total cost of the system to cover 65% of the load (solar fraction) is $20,000. The owner will pay a...
-
You are asked to prepare a 100.0 mL sample of a solution with a pH of 5.50 by dissolving the appropriate amount of a solute in water with pH = 7.00. Which of these solutes would you use, and in what...
-
Explain why [H 3 O + ] in a strong acid solution doubles as the total acid concentration doubles, whereas in aweak acid solution,[H 3 O + ] increases only by about a factor of 2.
-
Dinoo Mathur wishes to determine whether the $1,000 price asked for Stanco Manufacturings bond is fair in light of the theoretical value of the attached warrants. The $1,000-par-value, 30-year,...
-
Padgett invests in Bryant Inc., a glass manufacturing company. As a stockholder, he owns a part of the company and he holds the right to vote on company issues. However, he is entitled to dividends...
-
Feeling hungry, you decide to take a deep dive into data from your local town regarding estimated tons of oranges for each radius mile away from you (from 1 to 5). Optimistically, you believe that...
-
how to complete the Statement of Cash Flows ACC 122 Spring 2021 Comprehensive Project BestValue Corporation's Trial Balance at December 31, 20XX is presented below. All 20XX transactions have been...
-
d . The metal with the highest energy Fermi level from the options below is: i . Pb , Valency ( q ) = 4 , molar volume 1 8 . 2 6 cm - 3 ii . Al , Valency ( q ) = 3 , molar volume 1 0 cm - 3 iii. Zn ,...
-
I. Explain Requirements Engineering Process. I [04] [10 Marks] II. Explain System Modeling, its benefits and explain various types of modeling. [05] [10 Marks] III. Explain Prototyping and its role...
-
The following overhead data are for a department of a large company. Required Construct a flexible budget performance report that would be useful in assessing how well costs were controlled in...
-
Question 2 For an n x n matrix A = form) via (aij)
-
Boutique Ads Co. produces advertising videos. During the last six months of the current fiscal year, Boutique Ads Co. received the following notes: Instructions1. Determine for each note (a) The due...
-
The following data relate to notes receivable and interest for Vidovich Co Mar. 3. Received a $72,000, 9%, 60-day note on account. 25. Received a $10,000, 8%, 90-day note on account. May 2. Received...
-
The following were selected from among the transactions completed during the current year by Bonita Co., an appliance wholesale company: Jan. 20. Sold merchandise on account to Wilding Co., $30,750....
-
employees hired before 1996 who are paid retiring allowance have the option of transferring an eligible portion of the allowance to a RRSP or RPP with no income tax withholding at the source
-
ontinuous improvement violates which regression analysis assumption? Multiple choice question. The errors in estimating the costs are dependent on the cost drivers. The process for which costs are...
-
SP is a sustainable packaging company. They have developed a possibly innovative and (supposedly) environmentally friendly packaging product. They have decided to engage in an equity crowdfunding...

Study smarter with the SolutionInn App


