How much heat is required to raise the temperature of 215 g CH 3 OH(l) from 20.0
Question:
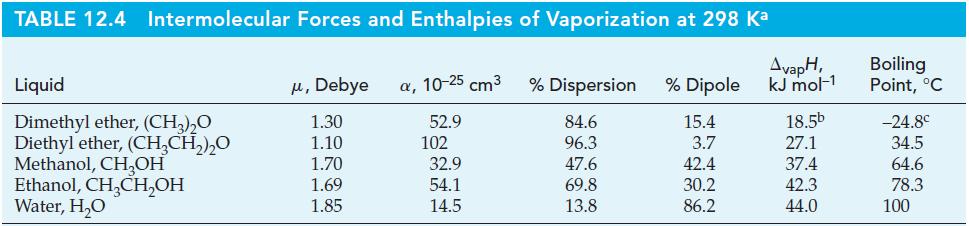
How much heat is required to raise the temperature of 215 g CH3OH(l) from 20.0 to 30.0 °C and then vaporize it at 30.0 °C? Use data from Table 12.4 and a molar heat capacity of CH3OH(l) of 81.1 J mol-1K-1.
Table 12.4
Transcribed Image Text:
TABLE 12.4 Intermolecular Forces and Enthalpies of Vaporization at 298 Ka Liquid Dimethyl ether, (CH₂)₂O Diethyl ether, (CH₂CH₂)₂0 Methanol, CH₂OH Ethanol, CH₂CH₂OH Water, H₂O μ, Debye α, 10-25 cm³ 1.30 52.9 1.10 1.70 1.69 1.85 102 32.9 54.1 14.5 % Dispersion % Dipole 84.6 15.4 96.3 3.7 47.6 42.4 69.8 30.2 13.8 86.2 AvapH, kJ mol-1 18.5b 27.1 37.4 42.3 44.0 Boiling Point, °C -24.8⁰ 34.5 64.6 78.3 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
Step 1 Heating the liquid from 200 to 300 C Heat required q1 mass m specific heat capacity c te...View the full answer

Answered By

Albert Kinara
i am an expert research writer having worked with various online platform for a long time. i also work as a lecturer in business in several universities and college part time and assure you well researched and articulate papers. i have written excellent academic papers for over 5 year and have an almost similar experience experting many clients in different units. bachelor of commerce (finance)
masters in strategic management
phd finance
4.60+
26+ Reviews
48+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Debye's T3 Law. At very low temperatures the molar heat capacity of rock salt varies with temperature according to Debye's T3law: C= kT3/O3 Where k = 1940J/mol' K and 0= 281 K. (a) How much heat is...
-
5 Match the items in List-I with items in List-II. List-I List-II a) Margin of Safety i) Earning Power b) ROI ii) Cash Flow statement c) Current Ratio iii) Break Even analysis d) Cash Equivalentsiv)...
-
A 0.400-kg aluminum teakettle contains 2.00 kg of water at 15.0C. How much heat is required to raise the temperature of the water (and kettle) to 100.0C?
-
The rigid bar AB is supported by a pin at B and by two the cables AC (perpendicular to the beam) and AD (inclined with respect to the beam) attached at A as shown in Fig. 3. A C 30 in D B 80 in...
-
United Road Machinery Company, a dealer in heavy road equipment (including truck scales supplied by Thurman Scale Company), received a telephone call on July 21 from James Durham, an officer of...
-
Testosterone is one of the most important male steroid hormones. When testosterone is dehydrated by treatment with acid, rearrangement occurs to yield the product shown. Propose a mechanism to...
-
Write a code to test a Gaussian pseudorandom number generator. If you do not have a canned generator available, write a generator based on the Box-Muller algorithm in Appendix I. Apply the following...
-
Horizon Cellular manufactures cell phones for exclusive use in its communication network. Management must select a circuit board supplier for a new phone soon to be introduced to the market. The...
-
Scenario 1: Joey is a 15 year old who is ready to work, but his parents want him to have more responsibility with money first. His parents have already set up checking and savings accounts for him at...
-
How many liters of CH 4 (g), measured at 23.4 C and 768 mmHg, must be burned to provide the heat needed to vaporize 3.78 L of water at 100 C? For CH 4 , comb H = -8.90 x 10 2 kJ mol -1 . For H 2...
-
A vapor volume of 1.17 L forms when a sample of liquid acetonitrile, CH 3 CN, absorbs 1.00 kJ of heat at its normal boiling point (81.6 C and 1 atm). What is vap H in kilojoules per mole of CH 3 CN?
-
In Exercises find the indefinite integral using any method. S cos x In(sin x) dx
-
Business income is income the taxpayer earn from : Question 6 options: A profession or A trade or A manufacture or undertaking of any kind All of the above Question 7 (1 point) Business income does...
-
Relate five levels of business ethics; companies use these levels for ethical reasoning and moral decision-making by using these levels, cross-function area professionals' moral responsibility and...
-
Communication is a major component of getting your ideas and findings across to your client. Yet, many times the consultant encounters resistance from the client, questioning the findings or...
-
Communication practices and any issues that likely to arise in the project. Briefly discuss the headings below: Communication dissemination including: Information type Purpose Frequency Channel Owner...
-
Briefly summarize 2-3 accounting and reporting procedures the PMHC Police Mental Health Collaboration Program uses to ensure the budgeting strategies are effective and transparent.
-
On January 1, Garcia Supply leased a truck for a four-year period, at which time possession of the truck will revert back to the lessor. Annual lease payments are $10,000 due on December 31 of each...
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
Payback Period concerning payback: a. Describe how the payback period is calculated, and describe the information this measure provides about a sequence of cash flows. What is the payback criterion...
-
Discounted Payback Concerning discounted payback: a. Describe how the discounted payback period is calculated, and describe the information this measure provides about a sequence of cash flows. What...
-
Average Accounting Return Concerning AAR: a. Describe how the average accounting return is usually calculated, and describe the information this measure provides about a sequence of cash flows. What...
-
Clonex Labs, Incorporated, uses the weighted-average method of process costing. The following data are available for one department for October: Units Percent Completed Materials Work in process,...
-
Griffin's Goat Farm, Incorporated, has sales of $654,000, costs of $305,000, depreciation expense of $39,000, interest expense of $34,000, and a tax rate of 23 percent. The firm paid out $118,000 in...
-
Old Country Links, Incorporated, produces sausages in three production departments-Mixing, Casing and Curing, and Packaging. In the Mixing Department, meats are prepared and ground and then mixed...

Study smarter with the SolutionInn App


