In an experiment similar to that described in Exercise 19, drop 1 carried a charge of 6.41
Question:
In an experiment similar to that described in Exercise 19, drop 1 carried a charge of 6.41 x 10-19 C; drop 2 had 1/2 the charge of drop 1; drop 3 had twice the charge of drop 1; drop 4 had a charge of 1.44 x 10-18 C; and drop 5 had 1/3 the charge of drop 4. Are these data consistent with the value of the electronic charge given in the text? Could Millikan have inferred the charge on the electron from this particular series of data? Explain.
Exercise 19
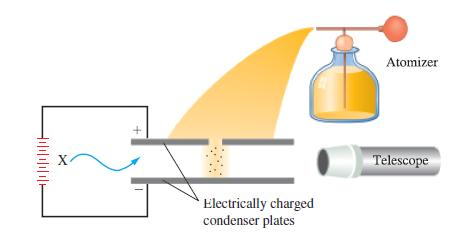
The following observations were made for a series of five oil drops in an experiment similar to Millikan’s (see Figure 2-8). Drop 1 carried a charge of 1.28 x 10-18 C; drops 2 and 3 each carried 1/2 the charge of drop 1; drop 4 carried 1/8 the charge of drop 1; drop 5 had a charge four times that of drop 1. Are these data consistent with the value of the electronic charge given in the text? Could Millikan have inferred the charge on the electron from this particular series of data? Explain.
Figure 2-8
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





