In Example 13-12, we used the vant Hoff equation to determine the temperature at which for the
Question:
In Example 13-12, we used the van’t Hoff equation to determine the temperature at which for the reaction 2 SO2(g) + O2(g) ⇌ 2 SO3(g). Obtain another estimate of this temperature with data from Appendix D and equations (13.13) and (13.17). Compare your result with that obtained in Example 13-12.
Example 13-12
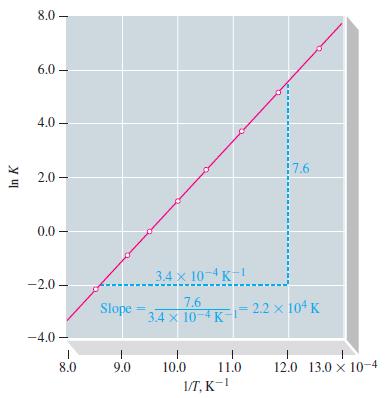
Use data from Table 13.8 and Figure 13-10 to estimate the temperature at which K = 1.0 x 106 for the reaction
![]()
Table 13.8
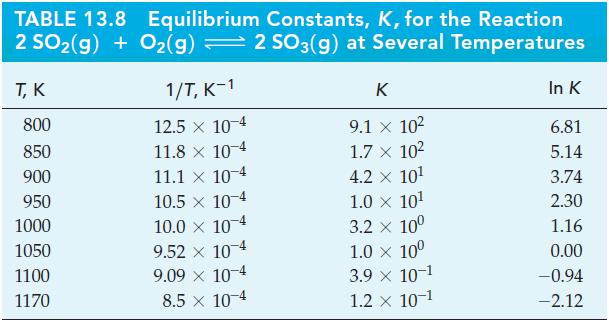
Figure 13-10
Eq. 13.13
![]()
Eq. 13.17
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





