In the Ostwald process for oxidizing ammonia, a variety of products is possibleN 2 , N 2
Question:
In the Ostwald process for oxidizing ammonia, a variety of products is possible—N2, N2O, NO, and NO2—depending on the conditions. One possibility is
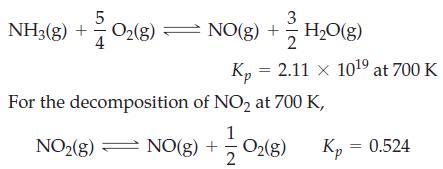
(a) Write a chemical equation for the oxidation of NH3(g) to NO2(g).
(b) Determine Kp for the chemical equation you have written.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





