Indicate which of the four cases in Table 13.3 applies to each of the following reactions. If
Question:
Indicate which of the four cases in Table 13.3 applies to each of the following reactions. If you are unable to decide from only the information given, state why.
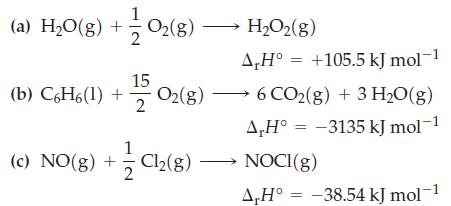
Table 13.3
Transcribed Image Text:
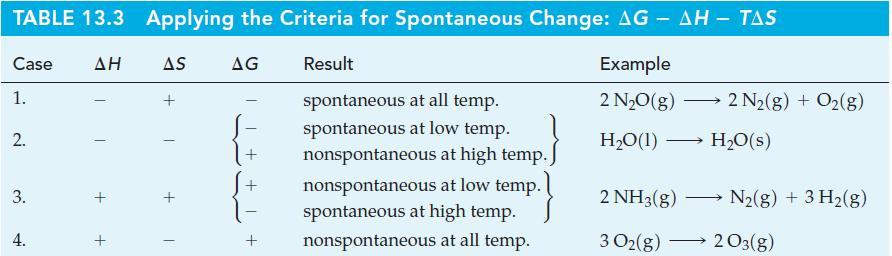
(a) H₂O(g) +0₂(g) → H₂O2(g) (b) C6H6(1) + 1/2O₂(8) (c) NO(g) + Cl₂(g) 2 A,H° +105.5 kJ mol-1 6 CO2(g) + 3 H₂O(g) A,H° -3135 kJ mol-1 NOCI(g) A,H°-38.54 kJ mol-¹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Indicate which of the four cases in Table 13.3 applies to each of the following reactions. If you are unable to decide from only the information given, state why. Table 13.3 (a) PC13(g) + Cl(g) (b)...
-
The following questions concern the determination of the proper sample size in audit sampling using the following table: Required a. Assume that the initial sample size for column 1 using...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
Evaluate the limit or state that it does not exist. lim (x,y) (1,-3) In(3x + y)
-
In what circumstances is time-and-material pricing most often used?
-
The Evanec Companys next expected dividend, D1, is $3.18; its growth rate is 6%; and its common stock now sells for $36.00. New stock (external equity) can be sold to net $32.40 per share. a. What is...
-
Design Data sold a piece of machinery to HHB Drafting Company. However, after HHB had taken possession of the machine, it discovered damage and revoked the contract. The court found that the...
-
Guillen, Inc. began work on a $7,000,000 contract in 2010 to construct an office building. Guillen uses the completed-contract method. At December 31, 2010, the balances in certain accounts were...
-
One way financial managers evaluate a firm's current financial condition is by computing ratios based on current accounts listed on the firm's financial statements. Financial managers look at four...
-
If a reaction can be carried out only because of an external influence, such as the use of an external source of power, which of the following changes in a thermodynamic property must apply? Explain....
-
Which of the following changes in a thermodynamic property would you expect to find for the reaction Br 2 (g) 2 Br(g) at all temperatures? Explain. (a) H < 0; (b) S > 0; (c) G < 0; (d) S < 0.
-
Why might projects within the same firm have different costs of capital?
-
Hermes, not to be confused with Hermes the French luxury goods manufacturer, is a pan-European courier company with over 40 years experience in the parcel delivery and courier business. It operates...
-
What are assets?
-
What are the weaknesses of the classification of economic agents according to the criterion of market and state?
-
When Will Shu, a former investment banker, spent long hours working in Londons Canary Wharf offices, he was forced to live off grocery store sandwiches for lack of an equally convenient option, he...
-
What sub-factors are included in the universal system of production factors?
-
Develop a privacy policy for database managers that provide a balance of consumer and seller perspectives. How would you encourage voluntary compliance with your policy? What methods of enforcement...
-
The water in tank A is at 270 F with quality of 10% and mass 1 lbm. It is connected to a piston/cylinder holding constant pressure of 40 psia initially with 1 lbm water at 700 F. The valve is opened,...
-
Life-cycle costing Fearless Furniture Manufacturing (FFM) has been manufacturing furniture for the home for over 30 years. George Fearless, the owner, has decided he would like to manufacture an...
-
Airline pricing, considerations other than cost in pricing. Air Americo is about to introduce a daily round-trip flight from New York to Los Angeles and is determining how it should price its...
-
Ethics and pricing. Baker, Inc., is preparing to submit a bid for a ball-bearings order. Greg Lazarus, controller of the Bearings Division of Baker, has asked John Decker, the cost analyst to prepare...
-
At January 1, Y3, Mapleton Corp.'s defined benefit pension plan, under IFRS, had a defined benefit obligation of $ 100,000, while the fair value of the plan assets was $ 120,000. During Y3, the...
-
Classified Electronics has an unfunded retiree health care plan. Each of the company's three employees has been with the firm since its inception at the beginning of 2023. As of the end of 2024, the...
-
Roberts Company had a beginning inventory balance 68,000Roberts cost of goods sold of $50,000 and had an ending inventory balance of $56,000, what was the goods available for sale for roberts this...

Study smarter with the SolutionInn App


