Organic chemists use a variety of methods to help them identify the functional groups in a molecule.
Question:
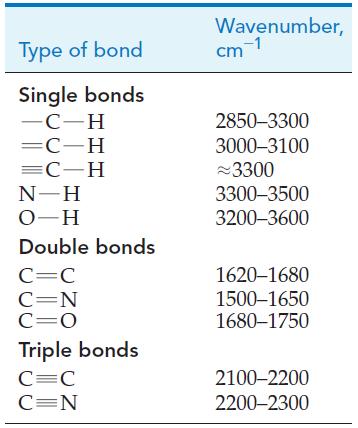
Organic chemists use a variety of methods to help them identify the functional groups in a molecule. In this chapter, we mentioned a few simple chemical ways to test for alkenes, alcohols, carboxylic acids, and so on. Such tests are quick and easy, but today organic chemists rely heavily on instrumental techniques. Infrared (IR) spectroscopy is an instrumental technique for identifying functional groups in a molecule. When infrared radiation is absorbed by a molecule, it causes atoms in bonds to vibrate back and forth with increased amplitude. We saw that the energies of electrons in atoms are quantized and so too are the vibrations of atoms in molecules. Because each functional group has a particular grouping of atoms, there is a characteristic infrared absorption associated with each type of functional group. Some characteristic infrared absorptions are summarized in the following table. Infrared absorptions of molecules are identified by specifying the wavenumber of the light that is absorbed. The wavenumber is simply the reciprocal of wavelength: wavenumber = 1/λ = ν/c. The SI unit for wavenumber is m-1 but it is often given in units of The wavenumber represents the number of cycles of the wave in each meter or centimeter along the light beam. The data in the table indicate that molecules containing cm-1. the carbonyl group (C = O) absorb light with wavenumbers between 1680 and 1750 cm-1. Molecules containing a carbon–carbon triple bond (C ≡ C) absorb light with wavenumbers between 2100 and 2200 cm-1. To identify the functional groups present in a molecule, the infrared spectrum of the molecule is obtained by using an instrument called an infrared spectrometer. A schematic diagram of an infrared spectrometer is shown below. The light from the infrared light source is directed through a monochromator, which can be set to select a specific wavelength of light. The light then passes through a beam splitter, which splits the light into two separate beams, a reference beam and an incident beam. If the wavenumber of the incident beam matches one of the characteristic absorptions of the molecule, the sample absorbs the light, producing molecules that vibrate with greater energy. Because light has been absorbed by the sample, the intensity of the transmitted beam is less than that of the reference beam. The decrease in intensity is detected by the detector. By varying the wavenumber of light that reaches the sample and monitoring the percentage of light that is transmitted, an infrared spectrum is obtained (see graphs). An infrared spectrum is a plot of percent transmittance versus wavenumber. One hundred percent transmittance means none of the incident light was absorbed and 0% transmittance means all of the incident light was absorbed.
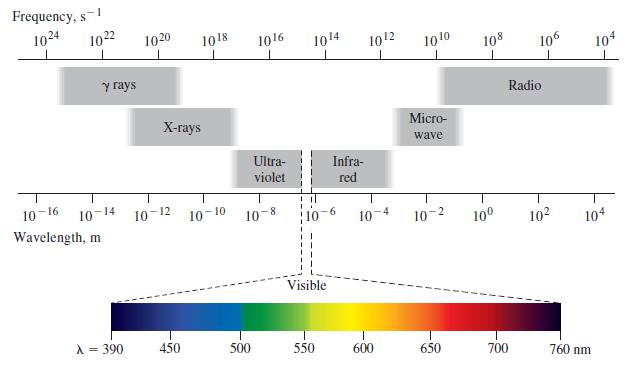
(a) The infrared absorptions given in the table above range from 1500 cm-1 to 3600 cm-1. Calculate the corresponding ranges of wavelength and frequency to verify that these absorptions correspond to the infrared region of the electromagnetic spectrum. (See Figure 8-3.)
(b) Identify the bonds responsible for the absorptions labeled A, B, C, and D in the two infrared spectra shown here. One spectrum is for acetone and the other is for 1-propanol, both of which are colorless liquids. Which of the two spectra is that of acetone?
(c) An isomer of acetone exhibits a strong IR absorption at 1645 cm-1 and also absorbs strongly from 2860 through 3600 cm-1. The compound decolorizes bromine water, Br2(aq), and produces bubbles of gas when sodium metal is added to it. What is the structure of this compound?
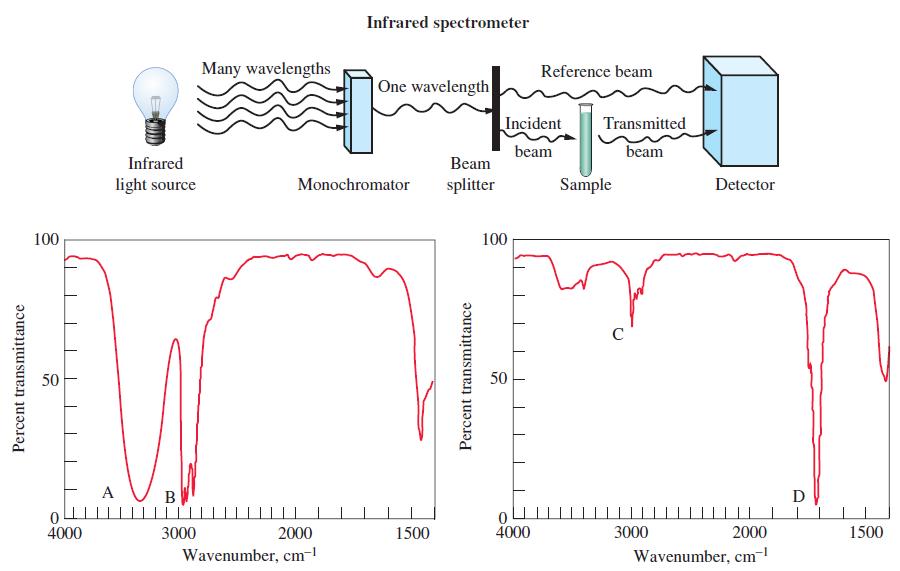
Figure 8-3
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




