R. S. Mulliken proposed that the electronegativity (EN) of an atom is given by where E i
Question:
R. S. Mulliken proposed that the electronegativity (EN) of an atom is given by
![]()
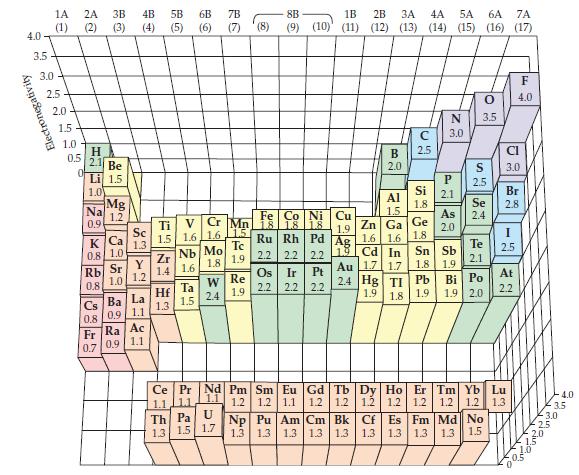
where Ei and Eea are the ionization energy and electron affinity of the atom, respectively. Using the electron affinities and ionization energy values for the halogen atoms up to iodine, estimate the value of k by employing the electronegativity values in Figure 10-6. Estimate the electron affinity of At.
Figure 10-6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





