Refer to Example 7-2. The experiment is repeated with several different metals substituting for the lead. The
Question:
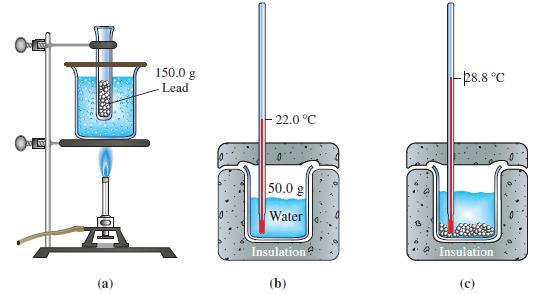
Refer to Example 7-2. The experiment is repeated with several different metals substituting for the lead. The masses of metal and water and the initial temperatures of the metal and water are the same as in Figure 7-3. The final temperatures are
(a) Zn, 38.9 °C;
(b) Pt, 28.8 °C;
(c) Al, 52.7 °C.
What is the specific heat capacity of each metal, expressed in J g-1 °C-1?
Example 7-2
Use data presented in Figure 7-3 to calculate the specific heat capacity of lead.
Figure 7-3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





