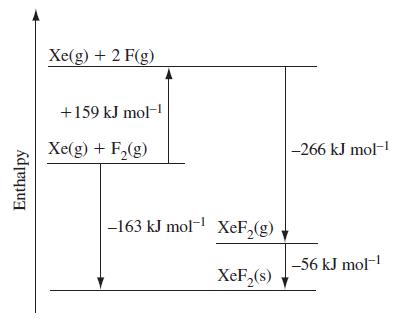
Refer to Figure 22-3 and then construct an enthalpy diagram for forming XeO 3 (g) from Xe(g)
Question:
Refer to Figure 22-3 and then construct an enthalpy diagram for forming XeO3(g) from Xe(g) and O2(g). For XeO3, the average bond enthalpy is about 36 kJ mol–1, and for O2, the bond enthalpy is 498 kJ mol–1 What is ΔfH° for XeO3(g)? Does your result support the observation that Xe(g) does not react directly with O2(g) to form XeO3(g)?
Figure 22-3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





