Question: Refer to Figure 9-11 and explain why the difference between the ionic radii of the -1 and -2 anions does not remain constant from top
Refer to Figure 9-11 and explain why the difference between the ionic radii of the -1 and -2 anions does not remain constant from top to bottom of the periodic table.
Figure 9-11
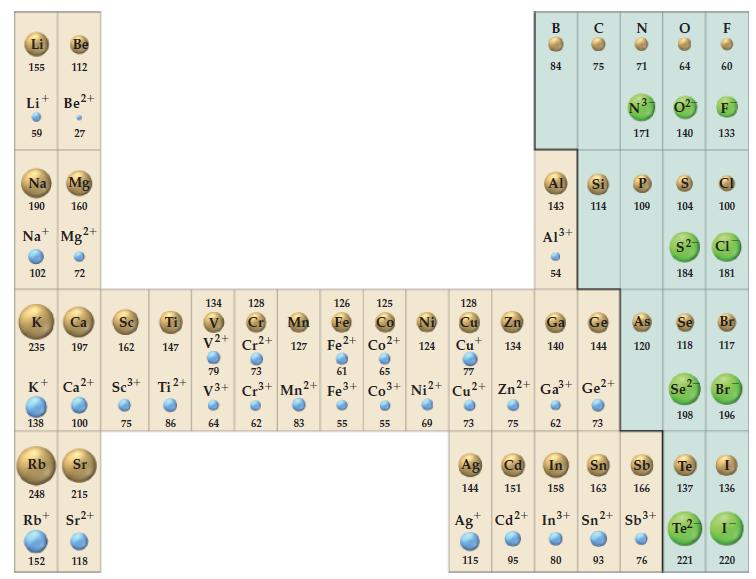
Li 155 Li+ Be+ 59 Na Mg 190 160 102 K 235 Be 112 Na Mg 138 27 Rb 2+ 152 K+ Ca+ Sc+ Ti + 72 Ca Sc Ti 197 162 147 100 Sr 248 215 Rb Sr+ 2+ 118 75 86 134 V V2+ 128 64 Cr2+ 79 73 V3+ Cr+ 62 Mn2+ 126 Fe Mn 127 Fe+ Co+ 83 61 Fe+ 125 55 65 124 55 128 77 Co+ Ni2+ Cu2+ Zn+ 69 Cu+ 73 Ag 144 115 75 B 84 95 Al 143 Zn Ga 134 140 A1+ 54 62 75 Ga+ Ge+ 80 144 Cd In Sn 151 158 163 Ag Cd2+ In+ Sn 2+ 73 ZO Si P 114 109 93 N 71 N3 171 120 Sb+ CO 76 3 64 S 0 140 133 104 $2 184 Se 118 2- Se Sb Te 166 137 198 Te F 221 60 100 CI 181 Br 117 Br 196 136 I 220
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
The difference between the ionic radii of the 1 and 2 anions does not remain constant from top to bo... View full answer

Get step-by-step solutions from verified subject matter experts


