Refer to the stability of [Co(NH 3 ) 6 ] 3+ (aq) (a) Verify that E cell
Question:
Refer to the stability of [Co(NH3)6]3+(aq)
(a) Verify that E°cell reaction (24.12) is +0.59 V.
(b) Calculate [Co3+] in a solution at equilibrium that has a total concentration of cobalt of 0.1 M and [NH3] = 0.1 M.
(c) Show that for the value of [Co3+] calculated in part (b), reaction (24.12) will not occur. Assume a low, but reasonable, concentration of Co2+ (say, 1 x 10–4 M) and a partial pressure of O2(g) of 0.2 atm.
Reaction (24.12)
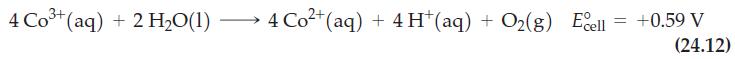
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





