Starting with 0.3500 mol CO(g) and 0.05500 mol COCl 2 (g) in a 3.050 L flask at
Question:
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of Cl2(g) will be present at equilibrium?
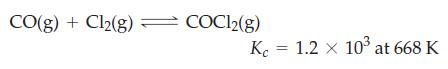
Transcribed Image Text:
CO(g) + Cl2(g) = COC1₂(g) Kc = 1.2 x 10³ at 668 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
1 Set up the ICE table Species Initial mol Change mol Equilibrium ...View the full answer

Answered By

Kalyan M. Ranwa
I have more than seven years of teaching experience in physics and mechanical engineering.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) If 0.150 mol H 2 (g) and 0.200 mol I 2 (g) are introduced into a 15.0 L flask at 445 C and allowed to come to equilibrium, how many moles of HI(g) will be present? (B) Suppose the equilibrium...
-
In the contact process, sulfuric acid is manufactured by first oxidizing SO2 to SO3, which is then reacted with water. The reaction of SO2 with O2 is A 2.000-L flask was filled with 0.0400 mol SO2...
-
A balloon with a volume of 1.50 L is at a pressure of 760 torr and a temperature of 30C. If the pressure is increased to 2300 torr and the temperature is raised to 72C, what is the new volume of the...
-
1.What is the difference between Router and firewall 2.What is Packet filtering router 3.What is Stateful inspection firewall 4.What is Circuit level firewall 5.What is Application level firewall...
-
JWG Company publishes Creative Crosswords. Last year the book of puzzles sold for $10 with variable operating cost per book of $8 and fixed operating costs of $40,000. How many books must JWG sell...
-
Jane Francois married Victor H. Francois. At the time of the marriage, Victor was a fifty-year-old bachelor living with his elderly mother, and Jane was a thirty-year-old, twice-divorced mother of...
-
In September 2013, Ugo Mattera entered into a written construction contract with Baja Properties, LLC. Stephen Chad Golden, the sole owner of Baja Properties, signed the contract and addendums on...
-
The following are independent internal controls commonly found in the acquisition and payment cycle. Each control is to be considered independently. 1. At the end of each month, an accounting clerk...
-
Oxygen gas having a volume of 1250 cm 3 at 43.1 C and 1.04 x 10 5 Pa expands until its volume is 1900 cm 3 and its press is 1.08 x 10 5 Pa. Find: (a) the number of moles of oxygen present and (b) the...
-
Equilibrium is established in a 2.50 L flask at 250 C for the reaction How many moles of PCl 5 , PCl 3 , and Cl 2 are present at equilibrium, if (a) 0.550 mol each of PCl 5 and PCl 3 are initially...
-
Starting with 0.280 mol SbCl 3 and 0.160 mol Cl 2 , how many moles of SbCl 5 , SbCl 3 , and Cl 2 are present when equilibrium is established at 248 C in a 2.50 L flask? SbC15(g) = SbCl3(g) + Cl(g) Kc...
-
Use either method to simplify each complex fraction. x + y 1 1 y X
-
Discuss the two key requirements for writing SQL Server audits to the Windows Security log. please provide 2 key references
-
You currently owe $2,500. If the bank charges 16.44% compounded semiannually and you want to pay off your debt in 3 years, how much must you pay every six months?
-
The formula = can be rewritten as 0= (ot. Using out for changes s=re to s=rot. Use the formula s=root to find the value of the missing variable. t= s = 6x cm, r = 1 cm, w = 2 radian per sec sec (Type...
-
Discuss how different Phishing Attacks work? What is biometrics and what are various ways to implement it?
-
Radiant heat makes it impossible to stand close to a hot lava flow. Calculate the rate of heat loss by radiation from 1.00 m 2 of 1230C fresh lava into 25.0C surroundings, assuming lava's emissivity...
-
Pathos Co.'s common stock is currently selling for $21.50. Dividends paid last year were $.70. Flotation costs on issuing stock will be 10 percent of market price. The dividends and earnings per...
-
With your classmates, form small teams of skunkworks. Your task is to identify an innovation that you think would benefit your school, college, or university, and to outline an action plan for...
-
Colliers Company has determined that the variable overhead rate is $2.90 per direct labor hour in the Fabrication Department. The normal production capacity for the Fabrication Department is 14,000...
-
The following data relate to factory overhead cost for the production of 5,000 computers: If productive capacity of 100% was 8,000 hours and the factory overhead cost budgeted at the level of 5,000...
-
Perma Weave Textiles Corporation began January with a budget for 30,000 hours of production in the Weaving Department. The department has a full capacity of 40,000 hours under normal business...
-
Find a peer - reviewed research article on the use of social marketing in public health. Summarize the message or article. And, identify who is the target audience? What is the main message it is...
-
The decision in McCullough v . Maryland, and in particular its effect on state power, is an example of what component of federalism at work?
-
Examples of an conclusion paragraph containing how managers can shape behavior using positive and negative reinforcement, locus of control , Maslow's need hierarchy, the three-needs theory, and the...

Study smarter with the SolutionInn App


