The accompanying drawing suggests a series of manipulations starting with saturated Mg(OH) 2 (aq). Calculate [Mg 2+
Question:
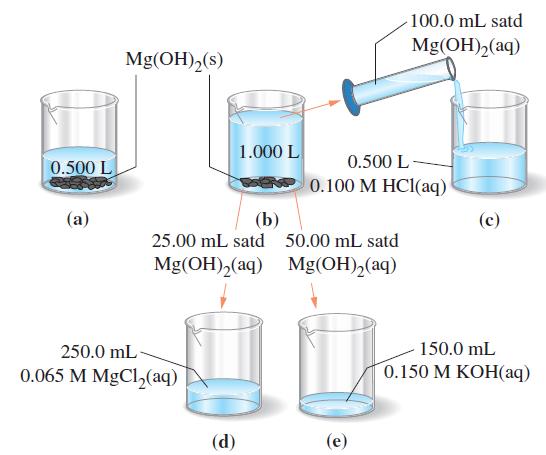
The accompanying drawing suggests a series of manipulations starting with saturated Mg(OH)2(aq). Calculate [Mg2+(aq)] at each of the lettered stages.
(a) 0.500 L of saturated Mg(OH)2(aq) is in contact with Mg(OH)2(s).
(b) 0.500 L of H2O is added to the 0.500 L of solution in part (a), and the solution is vigorously stirred. Undissolved Mg(OH)2(s) remains.
(c) 100.0 mL of the clear solution in part (b) is removed and added to 0.500 L of 0.100 M HCl(aq).
(d) 25.00 mL of the clear solution in part (b) is removed and added to 250.0 mL of 0.065 M MgCl2(aq).
(e) 50.00 mL of the clear solution in part (b) is removed and added to 150.0 mL of 0.150 M KOH(aq).
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





