The decomposition of HI(g) is represented by the equation HI(g) is introduced into five identical 400 cm
Question:
The decomposition of HI(g) is represented by the equation
![]()
HI(g) is introduced into five identical 400 cm3 glass bulbs, and the five bulbs are maintained at 623 K. Each bulb is opened after a period of time and analyzed for I2 by titration with 0.0150 M Na2S2O3(aq).
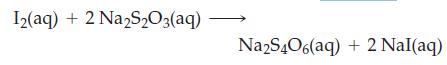
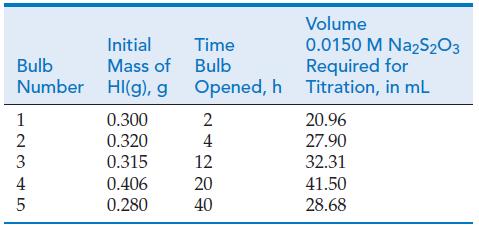
Data for this experiment are provided in the table. What is the value of Kc at 623 K?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





