The emission spectrum below is for hydrogen atoms in the gas phase. The spectrum is of the
Question:
The emission spectrum below is for hydrogen atoms in the gas phase. The spectrum is of the first few emission lines from principal quantum number 6 down to all possible lower levels.
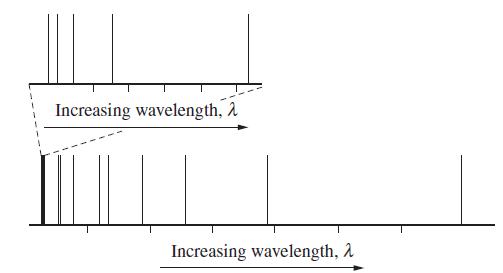
As discussed in Are You Wondering 8-6, not all possible de-excitations are possible; the transitions are governed by selection rules. Using the selection rules from Are You Wondering 8-6, identify the transitions, in terms of the types of orbital (s, p, d, ƒ), involved, that are observed in the spectrum shown above. In the presence of a magnetic field, the lines split into more lines according to the magnetic quantum number. Using the selection rule for mℓ, identify the line(s) in the spectrum that split(s) into the greatest number of lines.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





