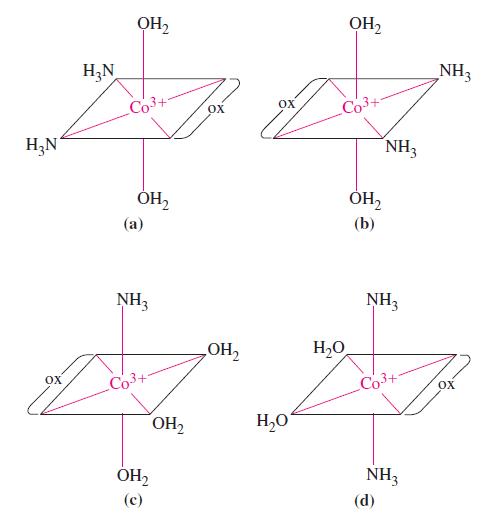
The structures of four complex ions are given. Each has Co 3+ as the central ion. The
Question:
The structures of four complex ions are given. Each has Co3+ as the central ion. The ligands are H2O, NH3, and oxalate ion, C2O42–. Determine which, if any, of these complex ions are isomers (geometric or optical); which, if any, are identical (that is, have identical structures); and which, if any, are distinctly different.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





