The two NaCl(aq) solutions pictured are at the same temperature. (a) Above which solution is the vapor
Question:
The two NaCl(aq) solutions pictured are at the same temperature.
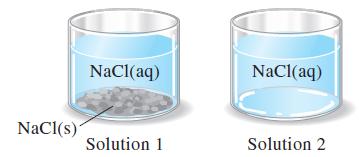
(a) Above which solution is the vapor pressure of water, PH2O, greater? Explain.
(b) Above one of these solutions, the vapor pressure of water, PH2O, remains constant, even as water evaporates from solution. Which solution is this? Explain.
(c) Which of these solutions has the higher boiling point? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





