Titanium dioxide, TiO 2 is the most widely used white pigment for paints, having displaced most lead-based
Question:
Titanium dioxide, TiO2 is the most widely used white pigment for paints, having displaced most lead-based pigments, which are environmental hazards. Before it can be used, however, naturally occurring TiO2 must be freed of colored impurities. One process for doing this converts impure TiO2(s) , to TiCl4(g) which is then converted back to pure TiO2(s). The process is based on the following reactions, the first of which generates TiCl4.
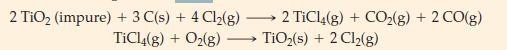
What mass of carbon is consumed in producing 1.00 kg of pure TiO2(s) in this process?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





