Use Coulombs law to verify the conclusion concerning the relative strengths of the attractive forces in the
Question:
Use Coulomb’s law to verify the conclusion concerning the relative strengths of the attractive forces in the ion pairs Na+Cl- and Mg2+O2- presented in Figure 12-36.
Figure 12-36
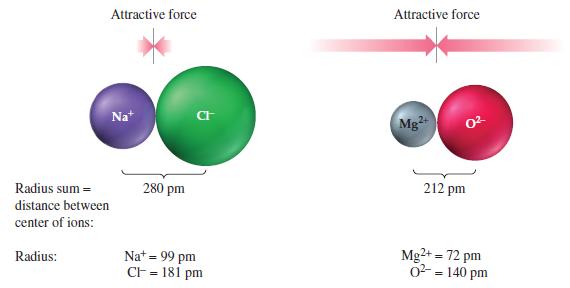
Transcribed Image Text:
Attractive force Radius sum= distance between center of ions: Radius: Nat 280 pm CI Na+ = 99 pm CF = 181 pm Attractive force Mg²+ 0²- 212 pm Mg2+ = 72 pm 0² = 140 pm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To verify the conclusion concerning the relative strengths of the attractive forces in the ion pairs NaCl and Mg2O2 presented in Figure 1236 using Cou...View the full answer

Answered By

Wonder Dzidzormenu
As a professional accountant and a teacher, I explain account ing concepts in a more practical way that makes students more connected to the subject.
With over 10 years of teaching accounting , I offer a well constructed , easily understood and in-depth explanations to students questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
Use Lenzs law to answer the following questions concerning the direction of induced currents. (a) What is the direction of the induced current in resistor R in Figure P31.28a when the bar magnet is...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Listed below are measured amounts of caffeine (mg per 12 oz of drink) obtained in one can from each of 20 brands (7-UP, A&W Root Beer, Cherry Coke, . . . , Tab). Are the statistics representative of...
-
James sells and delivers to Gerald on June 1 certain goods and receives from Gerald at the time of delivery Geralds check in the amount of $9,000 for the goods. The following day, Gerald is...
-
Draw a three-dimensional representation of the oxygen-bearing carbons atom in ethanol, CH3CH2OH, using the standard convention of solid, wedged, and dashed lines.
-
Refer to the information in Exercise 22-12. Assume that each of the companys divisions has a required rate of return of 7%. Compute residual income for each division. Data From Exercise 22-12 A food...
-
Anna Broderick is the dietitian for the State University football team, and she is attempting to determine a nutritious lunch menu for the team. She has set the following nutritional guidelines for...
-
Consider a 100-step binomial model where the price of a non-dividend-paying asset at time n, Sn is modelled as Sn = SoZ1 Z2... Zn where Z are i.i.d. random variables with u = 1.05 w. p. p Zi = d =...
-
Will the mineral villaumite (NaF) or periclase (MgO) have a higher Mohs hardness value (see Exercise 67)? Exercise 67 The hardness of crystals is rated based on Mohs hardness values. The higher the...
-
Are the fullerenes network covalent solids? What makes them different from diamond and graphite? It has been shown that carbon can form chains in which every other carbon atom is bonded to the next...
-
What is Comparative Negligence? If you were in a car accident, what conditions have to be met for you to recover under Comparative Negligence?
-
Fuel costs have risen quickly during recent years as consumption, refining and production costs have risen sharply. Supply and demand conditions in the perfectly competitive domestic crude oil market...
-
-Explain the OS design principles in terms of CPU Scheduling, Memory Management, and Deadlocks. -What are the CPU Scheduling, Memory Management algorithms, and Deadlock strategy used by the OS? -What...
-
Suppose the market for t-shirts has three potential sellers: Martin, whose willingness to sell is \$6, Adam, whose willingness to sell is $10, and Clara, whose willingness to sell is $12. If t-shirts...
-
Question 4 (1 point) You graduate and decide to start a new business with some of your classmates. The product your business makes is an App for students to take notes on ipad, which is called...
-
If you were to design an OS and RAM limitations were not an issue, how would you employ any two memory handling techniques from the list below? Would you take away any of them and why?
-
On January 1, 2013, Medical Transport Companys accumulated postretirement benefit obligation was $25 million. At the end of 2013, retiree benefits paid were $3 million. Service cost for 2013 is $7...
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
Calculating EAR a local finance company quotes a 15 percent interest rate on one-year loans. So, if you borrow $20.000, the interest for the year will be $3.000. Because you must repay a total of...
-
Calculating Present Values a 5-year annuity often $6,000 semiannual payments will begin 9 years from now, with the first payment coming 9.5 years from now. If the discount rate is 10 percent...
-
Calculating Annuities Due As discussed in the text, an ordinary annuity assumes equal payments at the end of each period over the life of the annuity. An annuity due is the same thing except the...
-
On January 1, 2024, the general ledger of Dynamite Fireworks includes the following account balances: Accounts Debit Cash Accounts Receivable Supplies Land Accounts Payable Common Stock Retained...
-
The following book and fair values were available for Beech Company as of June 1: Items Book Value Fair Value Inventory Land Buildings $ 406,000 817,500 2,005,000 $ 363,500 .1,087,500 2,314,750...
-
For this assignment, you will be given three scenarios and you must calculate the ROI using the payback period, net present value, and internal rate of return. Once you have performed the...

Study smarter with the SolutionInn App


