Question: Use data from Table 2.1 to verify that (a) The mass of electrons is about 1/2000 that of H atoms; (b) The mass-to-charge ratio (m/e)
Use data from Table 2.1 to verify that
(a) The mass of electrons is about 1/2000 that of H atoms;
(b) The mass-to-charge ratio (m/e) for positive ions is considerably larger than that for electrons.
Table 2.1
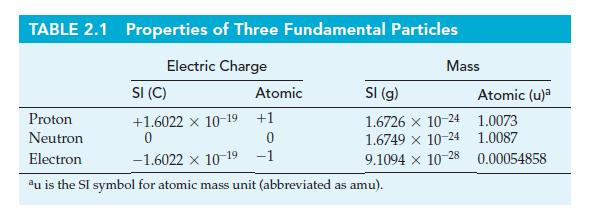
TABLE 2.1 Properties of Three Fundamental Particles Electric Charge SI (C) +1.6022 x 10-19 +1 0 Atomic Mass SI (g) 1.6726 x 10-24 1.6749 x 10-24 9.1094 x 10-28 Proton Neutron 0 Electron -1.6022 10-19 -1 au is the SI symbol for atomic mass unit (abbreviated as amu). Atomic (u)a 1.0073 1.0087 0.00054858
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
a Verify that the mass of ... View full answer

Get step-by-step solutions from verified subject matter experts


