Question: Use electrode potential data from this chapter or Appendix D to predict whether each of the following reactions will occur to any significant extent under
Use electrode potential data from this chapter or Appendix D to predict whether each of the following reactions will occur to any significant extent under standard-state conditions.
(a)
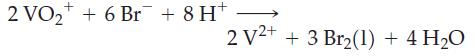
(b)
![]()
(c)
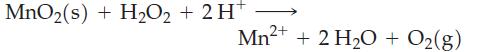
+ 2 VO + 6 Br + 8 H+ 2 V2+ + 3 Br(1) + 4 H0
Step by Step Solution
3.31 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


