Use the concept of hybrid orbitals to describe the bonding in the strong acids given in Exercise
Question:
Use the concept of hybrid orbitals to describe the bonding in the strong acids given in Exercise 78.
Exercise 78
The following very strong acids are formed by the reactions indicated:
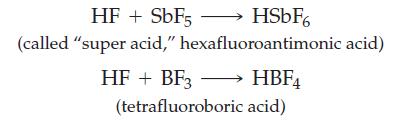
(a) Identify the Lewis acids and bases.
(b) To which atom is the H atom bonded in each acid?
Transcribed Image Text:
HSbF6 hexafluoroantimonic acid) HF + SbF5 (called "super acid," HF + BF3 →→→→→→→ HBF (tetrafluoroboric acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Hybrid Orbitals in Strong Acids a Identifying Lewis Acids and Bases 1 Hexafluoroantimonic Acid HSbF6 ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The Equity Premium Puzzle: Investments in equities (like stocks) yield substantially higher returns than investment in bonds. By itself, this is no surprisebecause stocks are riskier than bonds. What...
-
The A. J. Swim Team soon will have an important swim meet with the G. N. Swim Team. Each team has a star swimmer (John and Mark, respectively) who can swim very well in the 100- yard butterfly,...
-
Use the concept of cash flow insolvency over time and describe what could happen if the problem is temporary rather than permanent.
-
If A = -2 6 1 -7 1 then det (A) = an and A-1 =
-
In corporate settings, it is not unusual for firms to assess the financial viability of a business unit and decide whether to retain it within the corporation or divest it. The selling of units that...
-
Why is historical experience often a poor basis for establishing standards? Discuss.
-
Consider the following cash flow profile and assume MARR is 10 percent/year. a. Determine the IRR(s) for this project. b. Is this project economically attractive? EOY 0 1 2 3 4 5 6 NCF -$101 $411...
-
On January 1, 2017, the ledger of Accardo Company contains the following liability accounts. Accounts Payable ....... $52,000 Sales Taxes Payable....... 7,700 Unearned Service Revenue..... 16,000...
-
In return for an investment of $34318 in a fixed interest security, you will receive $134 at the end of each half year plus your money back on redemption in 12 years. You intend to deposit all the...
-
An aqueous solution of two weak acids has a stoichiometric concentration, c, in each acid. If one acid has a K a value twice as large as the other, show that the pH of the solution is given by the...
-
What is the pH of a solution that is 0.68 M H 2 SO 4 and 1.5 M HCOOH (formic acid)?
-
Last week Lisa had gross earnings of $541.30. Lisa receives a base salary of $475 and a commission on sales exceeding her quota of $5000. What is her rate of commission if her sales were $6560?
-
3. (a) What magnitude of magnetic field would make a beam of electrons, traveling to the right of this page at a speed of 4.8x106 m/s, go undeflected through a region where there is a uniform...
-
Write a few sentences about C# or Project Management. Did you learn anything new when researching these topics? Did you find a helpful site or article that you can share with class? Second Post:...
-
Conduct stock valuations for Tesla using a set of techniques such as the Single-Index market model and Discounted Dividend Model (DDM). Single-Index Market Model: The Single-Index market model is a...
-
Given the lines: and r = (4, -2, -3) + t(1, 5, 2) 1-2-9-5-2+1 y 3 -4 2 a) Determine if the lines are parallel or not. Explain your answer. b) Find the intersection of the two lines (if possible). c)...
-
Discuss the coverage of 5G technology by the leading telcos in Australia and their plans for future coverage. Can 5G technology help Australian businesses and services to increase efficiency and...
-
Discuss which approach to substantive testing you believe to be the most effective and indicate why. Create a way, other than using confirmations, for an auditing team to substantiate account...
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
Summit Industries has a past history of uncollectible accounts, as shown below. Estimate the allowance for doubtful accounts, based on the aging of receivables schedule you completed in Exercise 9-8....
-
Using data in Exercise 9-8, assume that the allowance for doubtful accounts for Summit Industries has a credit balance of $16,175 before adjustment on November 30. Journalize the adjusting entry for...
-
Fonda Bikes Co. is a wholesaler of motorcycle supplies. An aging of the company's accounts receivable on December 31, 2010, and a historical analysis of the percentage of uncollectible accounts in...
-
The functions f and g are defined as follows. 1(x) = 2431- g(x) = x+3x-4 2 X x +81 For each function, find the domain. Write each answer as an interval or union of intervals.
-
Convert to a logarithmic equation. 5-2 1 25
-
Solve this system of equations -3x-2y - 1x + 2y = 12 4

Study smarter with the SolutionInn App


