Use the unit cell of diamond in Figure 12-32(b) and a carbon-to-carbon bond length of 154.45 pm,
Question:
Use the unit cell of diamond in Figure 12-32(b) and a carbon-to-carbon bond length of 154.45 pm, together with other relevant data from the text, to calculate the density of diamond.
Figure 12-32(b)
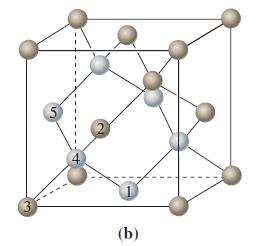
Transcribed Image Text:
(b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Calculating the density of diamond from the unit cell in Figure 1232b The unit cell of diamond is a ...View the full answer

Answered By

Samananda Khangembam
At present I do research in astrophysics at a university. Earlier I taught 10+2 physics at 2 premier Senior Secondary Schools in the state on Manipur in India. Besides, I also taught physics for test preparation for entry to medical and engineering schools in India. All together I have more than 5 years of experience in teaching physics at K2 level. I were also a teaching assistant for undergraduate studies at Northern Illoinois University(NIU), USA at the Department of Physics.
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The edge length of the unit cell of tantalum metal, Ta, is 330.6 pm; the unit cell is body centered cubic (one atom at each lattice point). Tantalum has a density of 16.69 g/cm3. What is the mass of...
-
A diamond unit cell is shown here. (a) How many carbon atoms are in one unit cell? (b) The unit cell can be considered as a cubic unit cell of C atoms with other C atoms in holes in the lattice. What...
-
Bugsy provided the following operating information during the current year. Operating income $ 185,000 Selling, general, and administrative costs 42,000 Cost of goods sold 77,500 Required: Provide...
-
In the previous problem, assume the equity increases by 1,500 solaris due to retained earnings. If the exchange rate at the end of the year is 1.24 solaris per dollar, what does the balance sheet...
-
Eldons Super Fresh Stores, Inc., is a corporation engaged in the retail grocery business. William Drexler was the attorney for and the corporate secretary of Eldons and was also the personal attorney...
-
Change to LIFO Retail Mueller Ltd., a local retailing concern in the Bronx, N.Y., has decided to change from the conventional retail inventory method to the LIFO retail method starting on January 1,...
-
Explain how the control variate technique is implemented.
-
Green Pastures is a 400-acre farm on the outskirts of the Kentucky Bluegrass, specializing in the boarding of broodmares and their foals. A recent economic downturn in the thoroughbred industry has...
-
1 Problem 4 - A firm's optimal output choice (10 points) Consider a price taking firm, where the market price for the output is given by P. The firm's output choice is denote by Q. The firm has a...
-
The enthalpy of formation of NaI(s) is -288 kJ mol -1 . Use this value, together with other data in the text, to calculate the lattice energy of NaI(s).
-
One way to describe ionic crystal structures is in terms of cations filling voids among closely packed anions. Show that in order for cations to fill the tetrahedral voids in a close packed...
-
Are the directors of a corporation agents of the shareholders?
-
Watch the video (https://youtu.be/16eWeGuo-Bg) in this week's folder about the disparity in sentencing when it comes to crack and cocaine. Once you've watched the video, answer these questions: 1....
-
The saving rate in Japan was unusually high in the 1980s. Gross saving was around 30 percent of GDP. Can such a high saving rate lead to sustained economic growth? Use the Solow-Swan model (and draw...
-
Virtually all economists and policymakers agree that, within limits, higher employment is better. If this is true, couldn't the government create more employment by hiring people to dig holes and...
-
How do evolutionary developmental biology (evo-devo) and molecular genetics shed light on the genetic mechanisms underlying the origin and diversification of species?
-
A country with a civilian population of 120,000 (all over age 16) has 100,000 employed and 10,000 unemployed persons, of which 5,000 are frictionally unemployed and another 3,000 structurally...
-
For each of the following inventory errors occurring in 2013, determine the effect of the error on 2013s cost of goods sold, net income, and retained earnings. Assume that the error is not discovered...
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
Statement of Cash Flows Suppose a company lengthens the time it takes to pay suppliers. How would this affect the statement of cash flows? How sustainable is the change in cash flows from this...
-
Calculating Liquidity Ratios SDJ, Inc., has net working capital of $l,570 current liabilities of $4,380, and inventory of $1,875. What is the current ratio? What is the quick ratio?
-
Calculating Profitability Ratios Country Boy, Inc., has sales of $24 million, total assets of $18 mil lion, and total debt of $7 million. If the profit margin is 8 percent, what is net income? What...
-
Simplify: 2x yz 6x-2yz
-
The Jonas had a really busy day, after leaving their home, Mrs. Jonas dropped son Cody at his tennis practice. She then drove her daughter Kristin 3 miles to her soccer game and stayed to watch....
-
As a newly qualified junior Mechanical Engineer working for a consultancy company offering research and expertise on green powered vehicles. You have been asked to analyse several scenarios relating...

Study smarter with the SolutionInn App


