Using the sketch in Exercise 61 as a guide, sketch the molecular view of a solution in
Question:
Using the sketch in Exercise 61 as a guide, sketch the molecular view of a solution in which
(a) HCl(aq) is titrated to the equivalence point with KOH(aq)
(b) CH3COOH(aq) is titrated halfway to the equivalence point with NaOH(aq).
Exercise 61
Which of the following points in a titration is represented by the molecular view shown in the sketch?
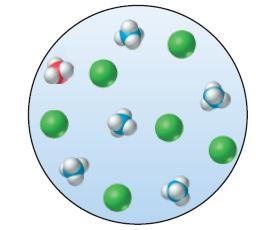
(a) 20% of the necessary titrant added in the titration of NH4Cl(aq) with HCl(aq)
(b) 20% of the necessary titrant added in the titration of NH3(aq) with HCl(aq)
(c) The equivalence point in the titration of NH3(aq) with HCl(aq)
(d) 120% of the necessary titrant added in the titration of NH3(aq) with HCl(aq).
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





