What volume of 18.5 C water must be added, together with a 1.23 kg piece of iron
Question:
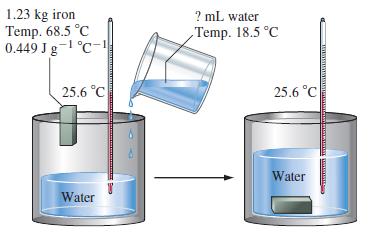
What volume of 18.5 °C water must be added, together with a 1.23 kg piece of iron at 68.5 °C, so that the temperature of the water in the insulated container shown in the figure remains constant at 25.6 °C?
Transcribed Image Text:
1.23 kg iron Temp. 68.5°C 0.449 Jg-1 °C-1 25.6 °C Water BASAARABALA ? mL water Temp. 18.5 °C 25.6 °C Water www MELATO TRATARE
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Q mcT where Q is the amount of heat transferred in Joules m is the mass of the substance in kilog...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
For the following exercises, graph the points and find a possible formula for the trigonometric values in the given table. 0 1 1 6 2 11 3 6 4 1 5 6
-
What volume of 18.5 C Water must be added together , with a 1.23 kg piece of iron at 68.5 C , so that the temprature of the water in the insulated container such as the temprature remains constant at...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
State with reasons, whether the following statements are true or false : (i) Overhauling expenses for the engine of motor car to get better fuel efficiency is revenue expenditure. (ii) Depreciation...
-
Assume that you are the managerial accountant at Infostore, a manufacturer of hard drives, CDs, and diskettes. Its reporting year-end is December 31. The chief financial officer is concerned about...
-
(a) If accounts payable are 55,000 and purchases are 232,000, what is the accounts payable/ purchases ratio? (b) How many days does the business take on average to pay its creditors?
-
Jack DeCoster owned Quality Egg, LLC, an Iowa egg production company. Jacks son, Peter DeCoster, served as the companys chief operating officer. Jack also owned and operated several egg production...
-
Contribution margin , gross margin, and margin of safety Mirabella Cosmetics manufactures and sells a face cream to small ethnic stores in the greater New York area. It presents the monthly operating...
-
You are a co-op student at Modus Biosystems and have been asked to research whether there are ways to save money on their legal liability insurance. Modus currently pays $75,000 a year for a policy...
-
Suppose that the point spread for a particular sporting event is 10 points and that with this spread you are convinced you would have a 0.60 probability of winning a bet on your team. However, the...
-
A British thermal unit (Btu) is defined as the quantity of heat required to change the temperature of 1 lb of water by 1 F. Assume the specific heat capacity of water to be independent of...
-
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH 3 CH(OH)COOH(s)]: f H = -694.0 kJ/mol. Table 7.2 TABLE...
-
Describe the minimum criteria for the creation of an administrative agency.
-
Explain whether Panda and CN are likely to recover the debts owing by Royal Thai. $320,000 owing to Panda Food Supplies Pte Ltd ("Panda"), an unsecured trade creditor $200,000 owing to Common Net Pte...
-
Dive into the challenges and best practices associated with reporting and disclosing intercompany transactions involving bonds and leases. What measures can companies adopt to enhance transparency...
-
Which is true regarding the taxation of social security benefits? Having earned income will reduce the tax on benefits RMD's are not considered when calculating any possible taxes owed Benefits are...
-
2 Suppose that a sphere of radius 1 is inscribed in a right circular cone with ra- dius and height h. (1) Express in terms of h. (2) Find the minimum of the volume of such a right circular cone.
-
In February 2020, Sally opened a joint bank account in the name of her sister Sue and herself. When she opened the account, Sally deposited $150,000. In March 2021, Sue withdrew $40,000 from the...
-
Refer to the note related to inventories in the CVS annual report in the Supplement to Chapter 16 to answer the following questions: What inventory method(s) does CVS use? Do you think many of the...
-
Dawson Companys balance sheet information at the end of 2019 and 2020 is as follows: Additional information: The company did not issue any common stock during 2020. Required : Next Level Fill in the...
-
Acquisition Costs of Trucks Shabbona Corporation operates a retail computer store. To improve delivery services to customers, the company purchases four new trucks on April 1, 2010. The terms of...
-
Purchase and Self-Constructed Cost of Assets Dane Co. both purchases and constructs various equipment it uses in its operations. The following items for two different types of equipment were recorded...
-
Treatment of Various Costs Allegro Supply Company, a newly formed corporation, incurred the following expenditures related to Land, to Buildings, and to Machinery and Equipment. Determine the amounts...
-
Convert the expression from radical form to exponential form. (5k+2)2 (5k+ 2)=
-
What are the roles of meiotic recombination and crossover formation in promoting genetic diversity, homologous chromosome segregation, and the generation of recombinant gametes, and how are these...
-
The graph below shows the work done on a 4.00 kg mass. If the mass starts at rest, find its speed at position 5.00 metres. 80.0 N 4 160 120 3.00 m 5.00 m force versus Lxbxh position 12 = 12380 (11.8...

Study smarter with the SolutionInn App


