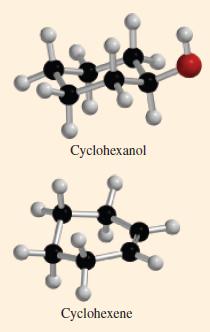
When heated with sulfuric or phosphoric acid, cyclohexanol, C 6 H 11 OH, is converted to cyclohexene,
Question:
When heated with sulfuric or phosphoric acid, cyclohexanol, C6H11OH, is converted to cyclohexene, C6H10. Ball-and-stick models of cyclohexanol and cyclohexene are shown here. The balanced chemical equation for the reaction is shown below.
![]()
If the percent yield is 83%, what mass of cyclohexanol must we use to obtain 25 g of cyclohexene?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





