Without doing a detailed calculation, state the outcome(s) you would expect in Figure 6-12(c) if an additional
Question:
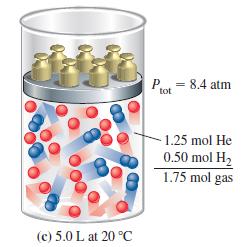
Without doing a detailed calculation, state the outcome(s) you would expect in Figure 6-12(c) if an additional 0.50 mol H2 were added to the cylinder in Figure 6-12(a):
(a) The mole fraction of H2 would double;
(b) The partial pressure of He would remain the same;
(c) The mole fraction of He would remain the same;
(d) The total gas pressure would increase by 50%;
(e) The total mass of gas would increase by 1.0 g.
Figure 6-12(a)
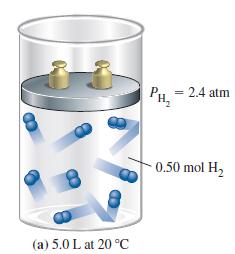
Figure 6-12(c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





