Question: Without performing any calculations or using data from Appendix D, predict whether r S for each of the following reactions is positive or negative.
Without performing any calculations or using data from Appendix D, predict whether ΔrS° for each of the following reactions is positive or negative. If it is not possible to determine the sign of ΔrS° from the information given, indicate why.
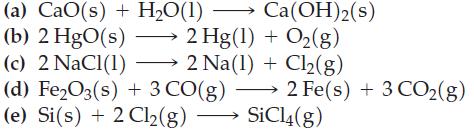
(a) CaO(s) + H2O(1) (b) 2 HgO(s) Ca(OH)2(s) 2 Hg(1) + O(g) (c) 2 NaCl(1) 2 Na(1) + Cl(g) (d) FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g) (e) Si(s) + 2 Cl(g) SiCl4(g)
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
a Both reactants and products are in condensed phases solid and liquid Since theres no change in the ... View full answer

Get step-by-step solutions from verified subject matter experts


