Without performing detailed calculations, determine which reaction produces the maximum quantity of O 2 (g) per gram
Question:
Without performing detailed calculations, determine which reaction produces the maximum quantity of O2(g) per gram of reactant.
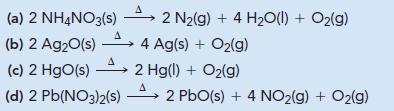
Transcribed Image Text:
2 N₂(g) + 4 H₂O(l) + O₂(g) (a) 2 NH4NO3(s) A (b) 2 Ag₂O(s) → 4 Ag(s) + O₂(g) A (c) 2 HgO(s) 2 Hg(l) + O₂(g) A (d) 2 Pb(NO3)2(s) 2 PbO(s) + 4 NO₂(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
The reaction that produces the maximum quantity of O2...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The main reaction of a charcoal grill is C(s) + O2(g) CO2(g). Which of the statements below are incorrect? Why? a. 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of CO2. b. 1...
-
Woodalt plc has two automated machine groups X and Y, through which timber is passed in order to produce two models of an item of sports equipment. The models are called ?Traditional? and...
-
16 Cornco produces two products: PS and QT. The sales price for each product and the maximum quantity of each that can be sold during each of the next three months are given in Table 16. Each product...
-
Henries Drapery Service is investigating the purchase of a new machine for cleaning and blocking drapes. The machine would cost $137,320, including freight and installation. Henries estimated the new...
-
Mansfield Company began operations on January 1, 2008, and purchased temporary investments in marketable securities during the year at a cost of $123,000. The end-of-period market value for these...
-
Alpha Division, which is part of the Delta Group, is considering an investment opportunity to which the following estimated information relates: 1. An initial investment of $45m in equipment at the...
-
Water flows through a two-dimensional diffuser having a \(20^{\circ}\) expansion angle as shown in Fig. P6.40. Assume that the flow in the diffuser can be treated as a radial flow emanating from a...
-
David Tennant has prepared the following list of statements about partnerships. 1. A partnership is an association of three or more persons to carry on as co-owners of a business for profit. 2. The...
-
Find f'(x) and find the equation of the line tangent to the graph of f at x = 3. f(x)= 5x 2* f'(x)=
-
(A) How many grams of magnesium nitride, Mg 3 N 2 , are produced by the reaction of 3.82 g Mg with an excess of N 2 ? (B) How many grams of H 2 are required to produce 1.00 kg methanol, CH 3 OH, by...
-
What mass of CO 2 is formed in the reaction of 4.16 g triethylene glycol, C 6 H 14 O 4 with an excess of O 2 ?
-
The Renfros were granted a decree of divorce in 2018 that was substantially revised in 2020. In accordance with the decree, Josh Renfro is to pay his ex-wife $24,000 a year until their only child,...
-
It is known that when Y = 0, the amount of consumption = 1500, every time additional income occurs, 60% will be consumed. Yeq or equilibrium income = 400. Determine: a) The size of the MPC and MPS b)...
-
Write a program that 1) calculates perimeter/circumference and area for specific geometric plane figures and 2) calculates the volume and surface area of specific geometric solids. The program should...
-
When it gets hot outside, the level of crime increases. Using this statement, discuss the problems of trying to measure such a vague statement. Discuss the progression of moving from vague terms to...
-
Olga owns 400 shares of stock which she would like to have the right to sell at $25 a share. The 25 call option is quoted at $.45 bid, $.50 ask. The 25 put is quoted at $.60 bid, $.65 ask. How much...
-
Company S has developed an industrial endoscope available to explore inner part of the decrepit water pipes. It is possible to explore the inner part of the pipes putting the endoscope in a certain...
-
Education reform is one of the most hotly debated subjects on both state and national policy makers list of socioeconomic topics. Consider a linear regression model that relates school expenditures...
-
Time Travel Publishing was recently organized. The company issued common stock to an attorney who provided legal services worth $25,000 to help organize the corporation. Time Travel also issued...
-
You are considering an investment in the common stock of Keller Corp. The stock is expected to pay a dividend of $2 a share at the end of the year (D1 = $2.00). The stock has a beta equal to 0.9. The...
-
What will be the nominal rate of return on a preferred stock with a $100 par value, a stated dividend of 8 percent of par, and a current market price of? (a) $60, (b) $80, (c) $100, and (d) $140?
-
Martell Mining Companys ore reserves are being depleted, so its sales are falling. Also, its pit is getting deeper each year, so its costs are rising. As a result, the companys earnings and dividends...
-
Read through the ethical scenario below. Refer back to prior chapters and your own individual research to answer part 1 as it relates to ethics. Then respond to each question listed below the...
-
Prepare a correct trial balance, assuming all account balances are normal.
-
Question: Below is the trial balance of N.A James as on October 1, 2019. Prepare income statement and balance sheet from the given trial balance. N.A James Trial Balance October 1, 2019 Accounts...

Study smarter with the SolutionInn App


