Question: Without performing detailed calculations, indicate whether equilibrium is displaced either far to the left or far to the right for each of the following reactions.
Without performing detailed calculations, indicate whether equilibrium is displaced either far to the left or far to the right for each of the following reactions. Use data from Appendix D as necessary.
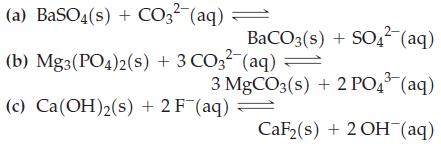
(a) BaSO4(s) + CO3-(aq) = (b) Mg3(PO4)2(s) + 3 CO3 (aq) = BaCO3(s) + SO4- (aq) 3 MgCO3(s) + 2 PO43 (aq) CaF(s) + 2OH(aq) (c) Ca(OH)2(s) + 2F (aq) =
Step by Step Solution
★★★★★
3.29 Rating (152 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


