Question: Write an acceptable value for each of the missing quantum numbers. (a) n = 3, (b) n (c) n = 4, l = (d) n
Write an acceptable value for each of the missing quantum numbers.
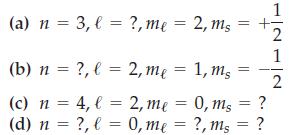
(a) n = 3, (b) n (c) n = 4, l = (d) n = ?, l= ?, l = ?, me = 2, ms 2, me = 1, ms 2, me 0, me = = 0, ms ?, ms ? = ? 1 1 2
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Acceptable values for the missing quantum numbers a n 3 l 2 ml 2 ms 12 b n 4 l 2 ml 1 ms 12 c n 4 l ... View full answer

Get step-by-step solutions from verified subject matter experts


