Write the equilibrium constant expression for each of the following reactions, and determine the value of K
Question:
Write the equilibrium constant expression for each of the following reactions, and determine the value of K at 25 °C. Use data from Table 19.1.
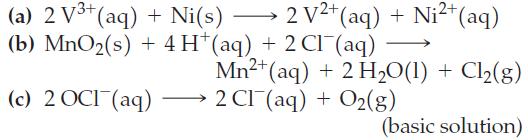
Table 19.1
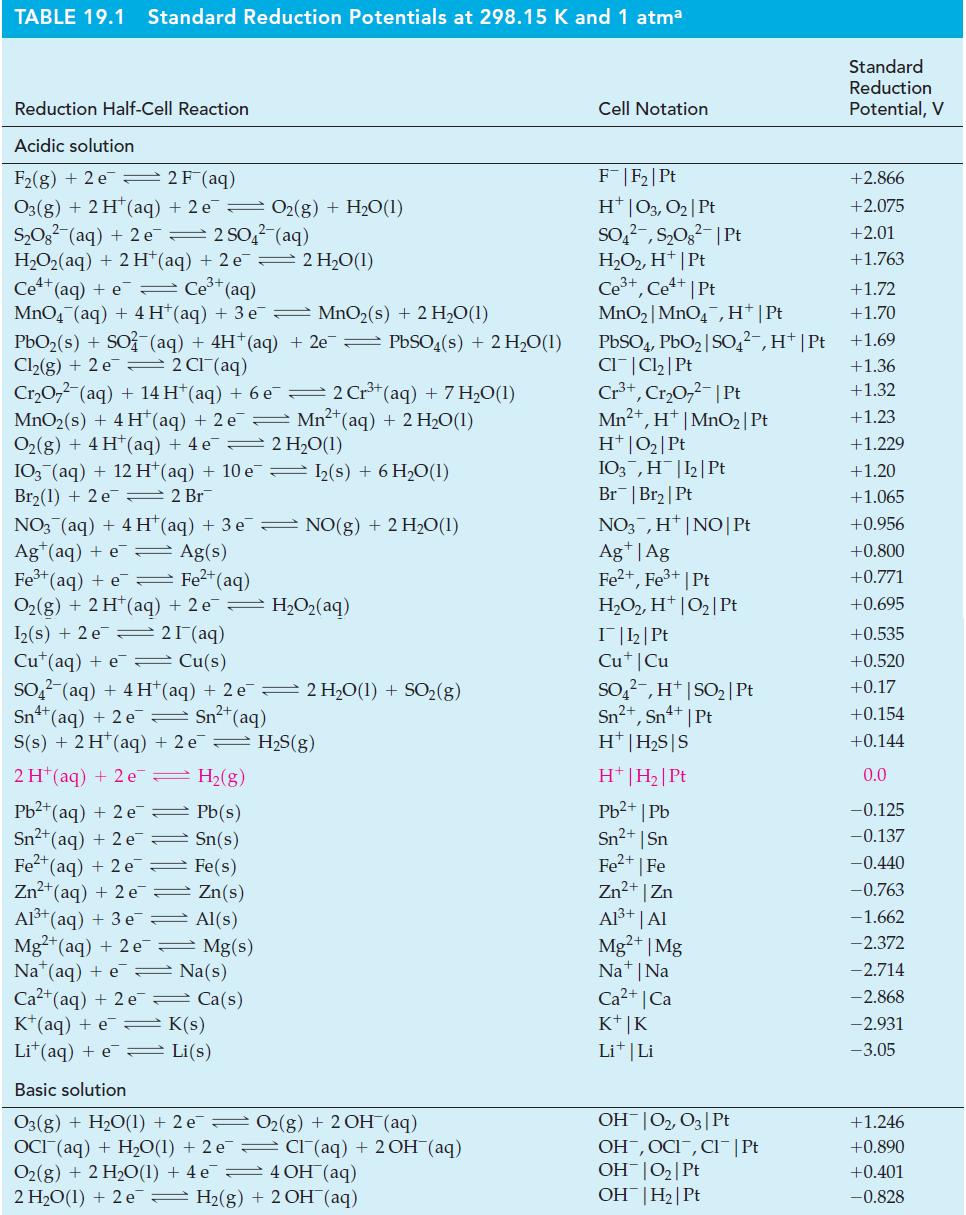
Transcribed Image Text:
2 V² (a) 2 V³+ (aq) + Ni(s) — > (b) MnO₂ (s) + 4 H*(aq) + 2 Cl(aq) (c) 2 OCI (aq) √2+ (aq) + Ni²+ (aq) Mn2+ (aq) + 2 H₂O(1) + Cl₂(g) 2 Cl(aq) + O2(g) (basic solution)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

WAHIDUL HAQUE
hello,
I'm a professional academic solution provider working as a freelance academic solution provider since 7 years. I have completed numerous projects. Help lots of students to get good marks in their exams and quizzes. I can provide any type of academic help to your homework, classwork etc, if you are a student of Accounting, Finance, Economics, Statistics. I believe in satisfying client by my work quality, rather than making one-time profit. I charge reasonable so that we make good long term relationship. why will you choose me? i am an extremely passionate, boldly honest, ethically driven and pro-active contractor that holds each of my clients in high regards throughout all my business relations. in addition, I'll always make sure that I'm giving my 100% better in every work that will be entrusted to me to be able to produce an outcome that will meet my client's standards. so if you are a student that is now reading my profile and considering me for your academic help. please feel free to look through my working history, feedback and contact me if you see or read something that interests you. I appreciate your time and consideration.
regards
4.90+
233+ Reviews
368+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Prove the identity for 0 1 k \ n |n k k 1
-
For the following exercises, find the determinant. 2 -3 -4 6 UWN 3 -5 1 1 1
-
Do you agree or not? Specialization of labor and better use of capital goods can initially generate increasing marginal output (returns) for a firm in the production of a good.
-
Based on the information collected in Question 22, how practical would it be to encourage foreign sales? Your average order ranges from about $250 to $800. All prices are quoted plus shipping and...
-
The actual cash received from cash sales was $6,973.60, and the amount indicated by the cash register total was $6,932.15. Journalize the entry to record the cash receipts and cash sales.
-
Mr. Jenkins, this is typical question: Do you feel that I have treated you fairly in this interview?
-
Treatment of Various Costs Allegro Supply Company, a newly formed corporation, incurred the following expenditures related to Land, to Buildings, and to Machinery and Equipment. Determine the amounts...
-
In some ways, a qualitative portfolio manager could never really be an index portfolio manager, whereas a quantitative portfolio manager could be. Explain why.
-
Write a cell diagram and calculate the value of E cell for a voltaic cell in which (a) Cl(g) is reduced to Cl(aq) and Fe(s) is oxidized to Fe+ (aq); (b) Ag (aq) is displaced from solution by Zn(s);...
-
In each of the following examples, sketch a voltaic cell that uses the given reaction. Label the anode and cathode; indicate the direction of electron flow; write a balanced equation for the cell...
-
It has been reported that women end up unhappier than men later in life, even though they start out happier (Yahoo News, August 1, 2008). Early in life, women are more likely to fulfill their family...
-
What are individual education programs/ plans (IEPs)? Who are individual education programs/ plans usually developed for? What should be included in an IEP?
-
Describe the authors approach to connecting with the audience What are three arguments made in this particular article Does this article seem like a reliable and current source? Why? Is the research...
-
What codes, standards or legislation may impact on the community services area you plan to work in? Explain briefly.
-
Provide five additional examples of the significance of business correspondence in the fields of Accountancy and business, besides the ones already mentioned (Inexpensive and Convenient, Helps in the...
-
What does the right of Freedom of Speech mean to you? Explain. Where do you think the future of Media & Media Technologies will be 20 years from now? Explain
-
How does vaccine distribution relate to key operations and supply chain management questions including: quality management, new product development and forecasting?
-
What is the role of business risk analysis in the audit planning process?
-
Identify five items that are adjustments to convert net income to net cash provided by operating activities under the indirect method.
-
Why is the statement of cash flows useful?
-
Describe the direct method for determining net cash provided by operating activities.
-
On Monday an electric bass caftsperson attaches fret to four necks and cuts three frames. Doing all of this takes a total of 7 hours. On Tuesday this same craftsperson spends a total of 8 hours and...
-
1. Considering the bar chart showing initial and final temperature, pressure, and volume for some ideal gas process, use the ideal gas law to help you draw the corresponding graph of pressure vs...
-
I created a survey example for a college assignment. I'm looking for someone to help (10 people) . Please take a few minutes to fill out this survey. The survey settings are fictitious. Link to page:...

Study smarter with the SolutionInn App


