You must estimate E for the half-cell reaction In 3+ (aq) + 3 e In(s). You
Question:
You must estimate E° for the half-cell reaction In3+(aq) + 3 e¯ → In(s). You have no electrical equipment, but you do have all of the metals listed in Table 19.1 and aqueous solutions of their ions, as well as In(s) and In3+(aq). Describe the experiments you would perform and the accuracy you would expect in your result.
Table 19.1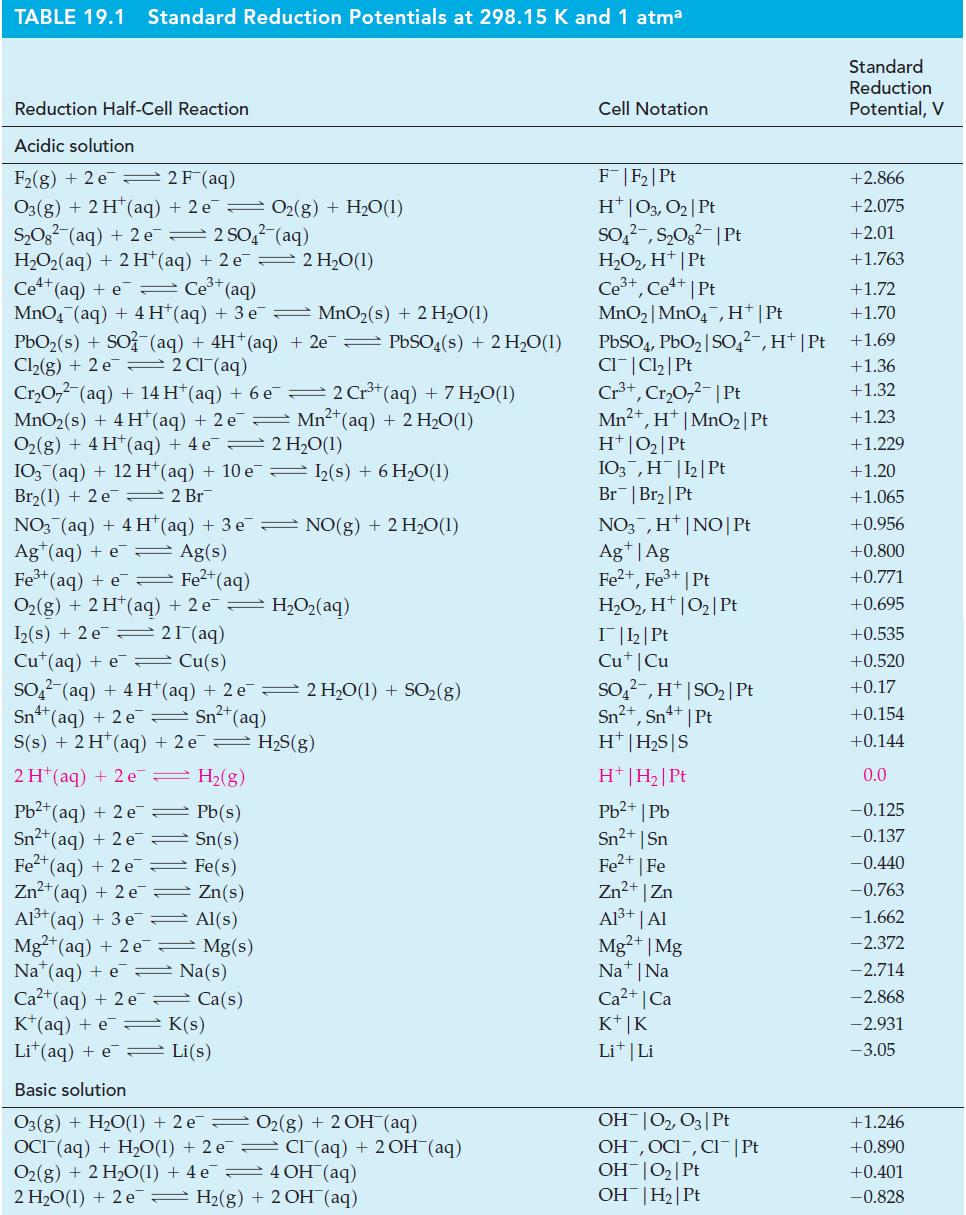
Transcribed Image Text:
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2 F (aq)
O3(g) + 2 H¹ (aq) + 2 e 0₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂(s) + SO² (aq) + 4H+ (aq) + 2e — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4 e
2 H₂O(1)
IO3(aq) + 12 H+ (aq) + 10 e
Br₂(1) + 2 e 2 Br
NO3 (aq) + 4 H(aq) + 3 e
Ag¹(aq) + e
Ag(s)
Zn(s)
Al(s)
= Mg(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma
Reduction Half-Cell Reaction
Acidic solution
F₂(g) + 2 e 2 F (aq)
O3(g) + 2 H¹ (aq) + 2 e 0₂(g) + H₂O(1)
S₂O² (aq) + 2 e
2 SO4²(aq)
H,Oz(aq) + 2H*(aq) +2e <2H,O(1)
Ce+ (aq) + Ce³+ (aq)
MnO₂ (aq) + 4H*(aq) + 3 e¯ ⇒ MnO₂(s) + 2 H₂O(1)
PbO₂(s) + SO² (aq) + 4H+ (aq) + 2e — PbSO(s) + 2 H₂O(1)
Cl₂(g) + 2 e 2 Cl(aq)
Cr₂O7² (aq) + 14 H*(aq) + 6 e¯ — 2 Cr³+ (aq) + 7 H₂O(1)
MnO2 (s) + 4H* (aq) + 2 e Mn²+ (aq) + 2 H₂O(1)
O₂(g) + 4H+ (aq) + 4 e
2 H₂O(1)
IO3(aq) + 12 H+ (aq) + 10 e
Br₂(1) + 2 e 2 Br
NO3 (aq) + 4 H(aq) + 3 e
Ag¹(aq) + e
Ag(s)
Zn(s)
Al(s)
= Mg(s)
Fe³+ (aq) + e
Fe²+ (aq)
Oz(g) +2H*(aq) +2e
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
To estimate the standard reduction potential E for the halfcell reaction Inaq 3 e InsI would perform the following experiments Construct a galvanic ce...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Multiple Choice Questions 1. If a company has a $25,000 reduction in sales and an increase of $7,000 in fixed costs with a contribution margin ratio of 34 percent, by how much will net income change?...
-
The following combined income and retained earnings statement, along with selected balance sheet data, is provided for Gemini Corporation: Instructions: 1. Using the direct method, compute the amount...
-
In a small group of three or four, discuss the issue of how to deal with audience questions in a presentation. For example, does your lecturer ask and invite questions during the lecture? Or do they...
-
Spot price of natural gas. The table shown in the next column lists the spot price of natural gas (in dollars per million Btu) between 2000 and 2020. a. Using 2000 as the base period, calculate and...
-
Effects of Burger Kings Current Liabilities on Its Statement of Cash Flows The following items are classified as current liabilities on Burger King Holdings, Inc.s balance sheets as of June 30, 2008,...
-
If a 90-day bill with a face value of $100,000 is issued at an annual discount rate of 6.75%, what will be the net proceeds of the issue after paying an acceptance fee of $100? A. $98,124.87 B....
-
From the observations listed, estimate the value of E for the half-cell reaction M 2+ (aq) + 2 e M(s). (a) The metal M reacts with HNO 3 (aq), but not with HCl(aq); M displaces Ag + (aq), but not Cu...
-
E cell = 0.201 V for the reaction What is E for the reduction of [PtCl 4 ] 2- to Pt in acidic solution? 3 Pt(s) + 12 Cl(aq) + 2NO3(aq) + 8 H*(aq) 3[PtCl4] (aq) + 2NO(g) + 4HO(1)
-
The production of a certain plastic calls for a mixture of bleach and ammonia. However, the presence of chlorine gas is highly undesirable. Based on the results of Experiments 1, 2, and 3, which of...
-
Use a substitution of the form u= ax + b to evaluate the following indefinite integral. S3x 3x+4 dx
-
Task 3 In order to support other staff to complete future risk assessments, produce a short-written report that explains. how hazards that become risks can be controlled the importance of fully...
-
let arr = [x => x + 5, x => 8, x => x * 2]; let b = X; let a = arr.reduce((acc, f) => acc + f(b), 0); If we know a is 28, what's the value of X?
-
2. (10 pts.) Identify the point symmetry elements of the structures for which the given directions are equivalent. Enumerate the elements (i.e., the individual symmetry operations) that make up the...
-
Theory Newton's second law can be written in a more general form as where is the momentum of system of N objects and is the net external force on the system. This relationship says that the rate at...
-
Investigate methods for determining optimum shapes of blanks for deep-drawing operations. Sketch the optimally shaped blanks for drawing rectangular cups, and optimize their layout on a large sheet...
-
A supermarket chain is interested in exploring the relationship between the sales of its store-brand canned vegetables (y), the amount spent on promotion of the vegetables in local newspapers (x1)...
-
Dye Company manufactures a single product. Annual production costs incurred in the manufacturing process are shown below for two levels of production. Instructions(a) Define the terms variable costs,...
-
The controller of Dugan Industries has collected the following monthly expense data for use in analyzing the cost behavior of maintenance costs. Instructions(a) Determine the fixed and variable cost...
-
Black Brothers Furniture Corporation incurred the following costs. 1. Wood used in the production of furniture. 2. Fuel used in delivery trucks. 3. Straight-line depreciation on factory building. 4....
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


