A 3.75-g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO 4 ,
Question:
A 3.75-g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO4, and this solution is titrated with 0.150 M K2Cr2O7 (potassium dichromate). If it requires 43.7 mL of potassium dichromate solution to titrate the iron(II) sulfate solution, what is the percentage of iron in the ore? The reaction is
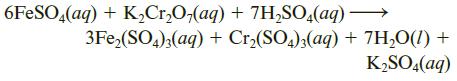
Transcribed Image Text:
6FESO,(aq) + K,Cr,O,(aq) + 7H,SO.(aq) → 3Fe,(SO,);(aq) + Cr,(SO,);(aq) + 7H,O(1) + K2SO,(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
First find the mass of Fe 2 required to react with the K 2 Cr 2 O 7 Pe...View the full answer

Answered By

Sabirah Shuaybi
I have diverse professional experience in teaching and tutoring - with all varieties of subjects ranging from Statistics, Computer Science/Programming, English as a Foreign Language and more. I am suited to working with all ages, from as young as 5 year olds to all the way up to the college level. I am looking forward to working with you and helping you achieve success in these subjects!
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 3.33-g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO4, and this solution is titrated with 0.150 M K2Cr2O7 (potassium dichromate). If it requires 43.7 mL of potassium...
-
A sample of iron ore (containing only Fe2+ ions) weighing 0.2792 g was dissolved in dilute acid solution, and all the Fe(II) was converted to Fe(III) ions. The solution required 23.30mL of 0.0194 M...
-
A solution of hydrogen peroxide, H2O2, is titrated with a solution of potassium permanganate, KMnO4. The reaction is 5H2O2(aq) + 2KMnO4(aq) + 3H2SO4(aq) 5O2(g) + 2MnSO4(aq) + K2SO4(aq) + 8H2O(l ) It...
-
In Exercises find the indefinite integral. sech(2x1) dx
-
Several years ago, a growing number of warranty claims on Firestone tires sold on Ford SUVs prompted Firestone and Ford to issue a major recall. An analysis of warranty claims data helped identify...
-
Consider 1.00 10 22 4 He atoms in a box of dimensions 1.0 cm 1.0 cm 1.0 cm. Calculate the occupancy of the first excited level at 1.0 mK, 2.0 K, and 4.0 K. Do the same for 3 He. What conclusions...
-
A rotary plug valve needs to be replaced to repair a machine, and the probabilities that the replacement will be a flange style (low pressure), flange style (high pressure), wafer style, or lug style...
-
Clarice Rich was employed by the New York City Board of Education as a clerk. It was her duty to prepare requisitions for checks to be issued by the board, to prepare the checks, to have them signed...
-
How does an organization strategize its crisis mitigation efforts amidst dynamic environmental factors and emergent complexities ?
-
A ball is kicked at a house window. The window is 4m up a vertical wall from a horizontal floor. The ball is kicked from a position that is 12 m distance from the foot of the wall. If the ball enters...
-
How many milliliters of 0.250 M KMnO 4 are needed to react with 3.55 g of iron(II) sulfate, FeSO 4 ? The reaction is as follows: 10FESO,(aq) + 2KMNO,(aq) + 8H,SO,(aq) 5Fe,(SO4);(aq) + 2MNSO,(aq) +...
-
Nickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Write the molecular equation for this reaction....
-
a. Consider the regression using three explanatory variables, FERTILITY, PUBLICEDUCATION, and LNHEALTH that you did in Exercise 3.3.6. Test whether PUBLICEDUCATION and LNHEALTH are jointly...
-
Evaluate lim x 3 7 sin () 2+5
-
Certain bacteria cells are being observed in an experiment. The population triples in 1 hour. If at the end of 3 hours, the population is 27,000, how many bacteria cells were present at the start of...
-
How do advanced theories of globalization, such as the global production network (GPN) framework, network theory, and world-systems theory, provide insights into the evolving dynamics of global...
-
TIGER ENTERPRISES Income Statement For the Year Ended December 31, 2024 ($ in thousands) Sales revenue $ 13,500 Operating expenses: Cost of goods sold $ 4,700 Depreciation expense 370 Insurance...
-
Jamie Lees father suggested that they purchase stock in a company that he has held shares in for decades.question They want to take advantage of the stock tip, but Jamie Lee and Ross are trying to...
-
Suppose that in a later market period, the quantities supplied in the table in Problem 3-1 are unchanged. The amount demanded, however, has increased by 30 million at each price. Construct the...
-
What are bounds and what do companies do with them?
-
A block of aluminum and a block of iron, both having the same mass, are removed from a freezer and placed outside on a warm day. When the same quantity of heat has flowed into each block, which block...
-
You have two samples of different metals, metal A and metal B, each having the same mass. You heat both metals to 95oC and then place each one into separate beakers containing the same quantity of...
-
Consider the reactions of silver metal, Ag(s), with each of the halogens: fluorine, F2(g), chlorine, Cl2(g), and bromine, Br2(l). What chapter data could you use to decide which reaction is most...
-
Jorgansen Lighting, Incorporated, manufactures heavy-duty street lighting systems for municipalities. The company uses variable costing for internal management reports and absorption costing for...
-
Assume that the stock of South Gate Corporations sells for R108 and is expected to increase by a factor of 1.25 or decline by a factor of 0.83 in 10 months. As a portfolio analyst, you find a call...
-
Use the below information to calculate the profit or loss of a short call butterfly spread if the spot price of the underlying asset at maturity is R89.45. June 70 Call costs R1.18 June 70 Put costs...

Study smarter with the SolutionInn App


