How many milliliters of 0.250 M KMnO 4 are needed to react with 3.55 g of iron(II)
Question:
How many milliliters of 0.250 M KMnO4 are needed to react with 3.55 g of iron(II) sulfate, FeSO4? The reaction is as follows: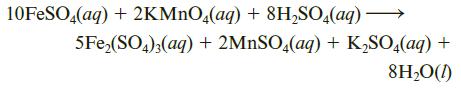
Transcribed Image Text:
10FESO,(aq) + 2KMNO,(aq) + 8H,SO,(aq) → 5Fe,(SO4);(aq) + 2MNSO,(aq) + K,SO,(aq) + 8H,O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The reaction is 10FeSO4 2KMnO4 8HSO...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many milliliters of 0.250 M KMnO4 are needed to react with 3.36 g of iron(II) sulfate, FeSO4? The reaction is as follows: 10FeSO4(aq) + 2KMnO4(aq) + 8H2SO4(aq) 5Fe2(SO4)3(aq) + 2MnSO4(aq) +...
-
For Reaction 1-7, how many milliliters of 0.165 0 M KMnO4 are needed to react with 108.0 mL of 0.1650 M oxalic acid? How many milliliters of 0.1650 M oxalic acid are required to react with 108.0 mL...
-
How many milliliters of 3.00 M H2SO4 are required to react with 4.35 g of solid containing 23.2 wt% Ba(NO3)2 if the reaction is Ba2++ SO24 BaSO4(s)?
-
In Exercises verify the identity. coshx = 1 + cosh 2x 2
-
One of the major measures of the quality of service provided by an organization is the speed with which the organization responds to customer complaints. A large family-held department store selling...
-
Calculate the electronic contribution to the molar internal energy at 1900 K for a sample composed of the atoms specified in Exercise 16.4a. Data in Exercise 16.4a. A certain atom has a threefold...
-
If each point of the sample space of Figure 3.12 represents an outcome having the probability find, 32
-
1. What is the macro and industry environment in the Southeast Asian region for the entrance of new budget airlines? What opportunities and challenges are associated with this environment? 2. How...
-
1. What is the difference between instrumental and terminal values? 2. Provide an example of how each of the Rokeach values might be linked with a brand's attributes and its benefits.
-
A graduated from the University in early 2021 at the age of 30. He immediately applied for a number of jobs and accepted a position as a financial planner in the Ottawa office of Otterbrook...
-
How many milliliters of 0.250 M H 2 SO 4 (sulfuric acid) are required to react with 8.20 g of sodium hydrogen carbonate, NaHCO 3 , according to the following equation? H 2 SO 4 (aq) + 2NaHCO 3 (aq) h...
-
A 3.75-g sample of iron ore is transformed to a solution of iron(II) sulfate, FeSO 4 , and this solution is titrated with 0.150 M K 2 Cr 2 O 7 (potassium dichromate). If it requires 43.7 mL of...
-
Describe the general procedure for conducting a t test.
-
What are the implications of demographic trends, such as population aging, urbanization, and migration, for labor markets, consumer demand patterns, and fiscal sustainability, and how do advanced...
-
Tommy Appleton is in charge of arranging the social hour period and dinner for the monthly meetings of the local chapter of the Management Accountants Association. Tommy is negotiating with a new...
-
Worldwide, t-shirt exports had a total value of $44,300,000,000 for a particular year. Of this amount, approximately (1)/(3) came from cotton t-shirts. Worldwide, what was the value of cotton...
-
A farmer plans to plant 270 acres of his farm with wheat and soybeans. For every 2 acres of land planted with soybeans, the farmer wants to plant 7 acres of land with wheat. The farmer uses the...
-
Describe the impact of both workforce trends and industry trends on I/O psychology.
-
For each of the following shifts in the demand curve and associated price change of a complement or substitute item, explain whether the price of the complement or substitute must have increased or a...
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
A 250-g sample of water at 20.0oC is placed in a freezer that is held at a constant temperature of 20.0oC. Considering the water as the system, answer the following questions: a. What is the sign of...
-
A 20.0-g block of iron at 50.0oC and a 20.0 g block of aluminum at 45oC are placed in contact with each other. Assume that heat is only transferred between the two blocks. a. Draw an arrow indicating...
-
What is the enthalpy change for the preparation of one mole of liquid water from the elements, given the following equations? H2(g) +-02(g)--H20(g):AH, H20(1)--H20(g); ,up
-
Using the data above, fill out the missing information below: The average of the variable score is 45 The average of the variable submission is 1.2 The sample variance of the submission is 1.7 . The...
-
Financial information is presented here for two companies. (a) Fill in the missing amounts. Sales revenue Sales returns and allowances (a) Crane Company Bramble Company $94,000 (d) $ 5,400 87,000...
-
Hartford Research issues bonds dated January 1 that pay interest semiannually on June 30 and December 31. The bonds have a $40,000 par value and an annual contract rate of 10%, and they mature in 10...

Study smarter with the SolutionInn App


