Methanol, CH 3 OH, is prepared industrially from the gas-phase catalytic balanced reaction that has been depicted
Question:
Methanol, CH3OH, is prepared industrially from the gas-phase catalytic balanced reaction that has been depicted here using molecular models.
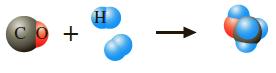
In a laboratory test, a reaction vessel was filled with 41.1 g CO and 10.2 g H2. How many grams of methanol would be produced in a complete reaction? Which reactant remains unconsumed at the end of the reaction? How many grams of it remain?
H Co+
Step by Step Answer:
First determine whether CO or H 2 is the limiting reactant by calculatin...View the full answer



Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
Methanol, CH3OH, is prepared industrially from the gasphase catalytic balanced reaction that has been depicted here using molecular models. In a laboratory test, a reaction vessel was filled with...
-
Methanol is prepared industrially from synthesis gas (CO and H 2 ). CO(g) + 2H 2 (g) CH 3 OH(g); H = 21.7 kcal Would the fraction of methanol obtained at equilibrium be increased by raising the...
-
Methanol is prepared industrially from synthesis gas (CO and H 2 ). CO(g) + 2H 2 (g) CH 3 OH(g); H = 21.7 kcal Would the fraction of methanol obtained at equilibrium be increased by raising the...
-
When a cosmetic manufacturer tests the market to determine how many women will buy eyeliner that has been tested for safety without subjecting animals to injury, is it involved in a descriptive...
-
Kayla sold a total of 145 Italian sausages and hot dogs from her curbside pushcart and collected $242.05. She sold 45 more hot dogs than sausages. How many of each did she sell? CENTR
-
How is stream processing different from feedback loop processing?
-
The Wide World of Fluids article titled "Galloping Gertie,". The Tacoma Narrows Bridge failure is a dramatic example of the possible serious effects of wind-induced vibrations. As a fluid flows...
-
Souder, Oles, and Franek is an international consulting firm headquartered in Chicago, Illinois. The Entity-Relationship diagram in Figure shows a simplified version of the companys process for...
-
Each of the four independent situations below describes a finance lease in which annual lease payments are payable at the beginning of each year. The lessee is aware of the lessor's implicit rate of...
-
A $25,000, 91-day Province of Newfoundland Treasury bill was originally purchased at a price that would yield the investor a 1.438% rate of return if the T-bill is held until maturity. Thirty-four...
-
Nickel(II) chloride reacts with sodium phosphate to precipitate nickel(II) phosphate. 3NiCl 2 (aq) + 2Na 3 PO 4 (aq) Ni 3 (PO 4 ) 2 (s) + 6NaCl(aq) How many moles of nickel(II) chloride are needed...
-
Calcium carbide, CaC 2 , used to produce acetylene, C 2 H 2 , is prepared by heating calcium oxide, CaO, and carbon, C, to high temperature. CaO(s) + 3C(s) CaC 2 (s) + CO(g) If a mixture contains...
-
Explain the "bottom line" on the meaning of the Bayh-Dole Act to research institutions. In what ways do you think the Act influenced the spirit of entrepreneurship at college campuses?
-
Two countries have populations of about 11.7 million and 767,000, respectively. Their areas are 8868 and 596,070 square miles, respectively. Compute the population densities of the two countries. The...
-
Describe other economic characteristics facing the Contract Management Food/Facilities industry (e.g. utility, normal versus luxury goods, etc.). Explain. Describe non-economic factors that face the...
-
George Halvorson declares that "health care is the epitome of a nonsystem" (2009, p. 2). Response to the following: Explain what is meant by this statement. What major factors are inhibiting health...
-
1. A force F = 20 + 10y acts on a particle in y-direction where F is in newton and y in meter. Work done by this force to move the particle from y = 0 to y = 1 m is : (1) 30 J (2) 5 J (3) 25 J (4) 20...
-
Most businesses sell several products at varying prices. The products often have different unit variable costs. Thus, the total profit and the breakeven point depend on the proportions in which the...
-
Kareem bought a rental house in March 2010 for $300,000, of which $50,000 is allocated to the land and $250,000 to the building. Early in 2012, he had a tennis court built in the backyard at a cost...
-
As water moves through the hydrologic cycle, water quality changes are common because of natural phenomena or anthropogenic pollution. Using Figure 11.1, describe how water-quality changes occur...
-
Classify each of the following reactions as a combination reaction, decomposition reaction, displacement reaction, or combustion reaction. a. When solid calcium oxide, CaO, is exposed to gaseous...
-
Consider the reaction of all pairs of the following compounds in water solution: Ba(OH)2, Pb(NO3)2, H2SO4, NaNO3, MgSO4. a. Which pair (or pairs) forms one insoluble compound and one soluble compound...
-
Consider the reaction of all pairs of the following compounds in water solution: Sr(OH)2, AgNO3, H3PO4, KNO3, CuSO4. a. Which pair (or pairs) forms one insoluble compound and one soluble compound...
-
What innovative approaches can organizations employ to reconcile competing demands for capital investment and operational expenditure within the constraints of finite budgetary resources, while still...
-
What measures can organizations implement to promote transparency and accountability in the budgeting process, thereby fostering trust and buy-in among stakeholders and facilitating...
-
Based on the following cost expenditures for the construction of an office / warehouse development ( to include direct and indirect charges ) , calculate the peak financial requirement: Month Monthly...

Study smarter with the SolutionInn App


