The solubility of a salt in water depends on a broad range of intermolecular bonding forces. These
Question:
The solubility of a salt in water depends on a broad range of intermolecular bonding forces. These occur between the particles or ions making up the salt, between the salt’s particles and solvating water molecules, and between water molecules themselves. Although the solubility of many salts tends to increase with temperature, this is not always the case. Take sodium chloride as an example. It’s solubility in water at 25°C is 35.89 g per 100 g H2O, and over the temperature range of 0°C to 100°C the solubility modestly ranges upwards from 35.65 g to 38.99 g per 100 g H2O. Still, the solubility of some salts decreases with temperature. Below is a table giving the solubility (in g salt/100 g H2O) for a few selected salts over the 0°C to 80°C temperature range. Follow the steps below to prepare the graphs (you can use Excel or other graphing tool) and answer the questions.
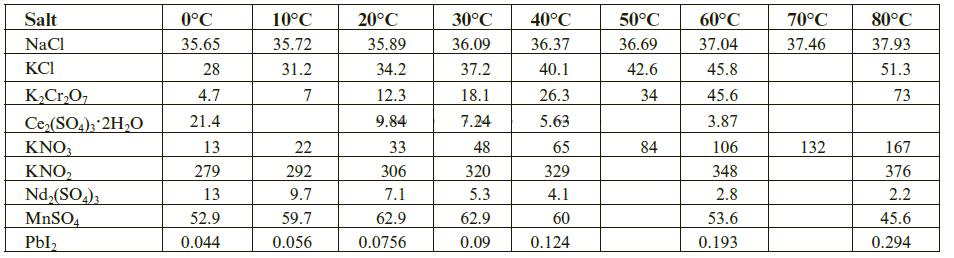
Step 1: Prepare a plot of solubility (y-axis) vs. temperature (oC) using the tabulated data above.
Step 2: Rescale the vertical axis to a maximum of 100 g/100 g H2O.
Step 3: Rescale the vertical axis to a maximum of 25 g/100 g H2O.
Step 4: Answer the following questions by reading the prepared graphs.
a. Many substances dissolve in water and absorb heat when doing so. This is referred to as an endothermic process and will be discussed in more detail in later chapters. For now, simply recognize that these processes are further favored as the temperature is increased.
For the chosen substances, which ones show an upward trend in solubility over the selected temperature range?
NaCl, KCl, K2Cr2O7, Ce2(SO4)3 · 2H2O, KNO3, KNO2, Nd2(SO4)3, MnSO4, PbI2
b. Other substances dissolve in water and release heat when doing so. This is referred to as an exothermic process and will be discussed in more detail in later chapters. For now, simply recognize that these processes are less favored as the temperature is increased. For the chosen substances, which show a downward trend in solubility over the selected temperature range?
NaCl, KCl, K2Cr2O7, Ce2(SO4)3 · 2H2O, KNO3, KNO2, Nd2(SO4)3, MnSO4, PbI2
c. Still other substances show more unusual solubility behaviors as the temperature is changed. For the chosen substances, which show neither a significantly upward or significantly downward trend in solubility over the selected temperature range?
NaCl, KCl, K2Cr2O7, Ce2(SO4)3 · 2H2O, KNO3, KNO2, Nd2(SO4)3, MnSO4, PbI2
Step 5: Prepare a scatter plot of the tabulated data. Note that some solubility data is missing in the original data set.
a. Use an appropriately scaled scatter plot to estimate the following solubilities:
solubility of Ce2(SO4)3·2H2O at 10oC solubility of Ce2(SO4)3·2H2O at 50oC solubility of KNO2 at 50oC
b. Many substances have such low solubility they are considered insoluble for all intents and purposes in the earlier discussions of aqueous phase chemistry reactions. Which of the chosen substances would you consider to be insoluble in this context?
NaCl, KCl, K2Cr2O7, Ce2(SO4)3 · 2H2O, KNO3, KNO2, Nd2(SO4)3, MnSO4, PbI2
Step by Step Answer:






