Question: The table below is partially completed for subshells A and B, where subshell B is the next subshell higher in energy compared to subshell A.
The table below is partially completed for subshells A and B, where subshell B is the next subshell higher in energy compared to subshell A. For example, if subshell A is the 4s subshell, then subshell B would be the 3d subshell. Use this criteria and the information provided to complete the table.
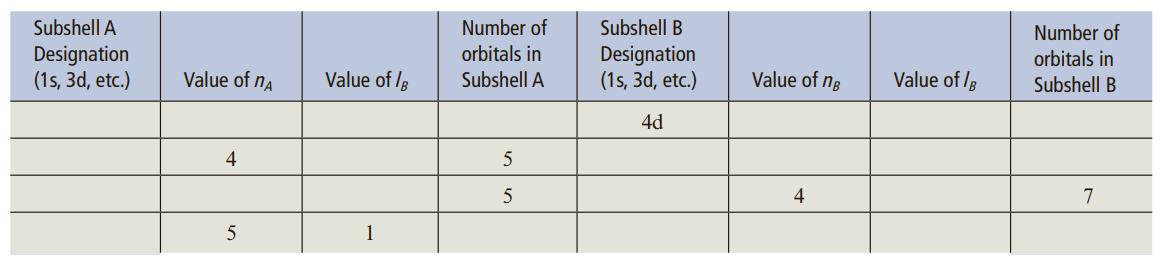
Number of orbitals in Subshell A Subshell A Subshell B Number of Designation (1s, 3d, etc.) Designation (1s, 3d, etc.) orbitals in Subshell B Value of na Value of /B Value of ng Value of /B 4d 4 5 4 7 1
Step by Step Solution
3.47 Rating (167 Votes )
There are 3 Steps involved in it
Determining the subshells A and B is key to completing the table Once one of these is found the othe... View full answer

Get step-by-step solutions from verified subject matter experts


