The table below is partially completed for subshells A and B, where subshell B is the next
Question:
The table below is partially completed for subshells A and B, where subshell B is the next subshell higher in energy compared to subshell A. For example, if subshell A is the 4s subshell, then subshell B would be the 3d subshell. Use this criteria and the information provided to complete the table.
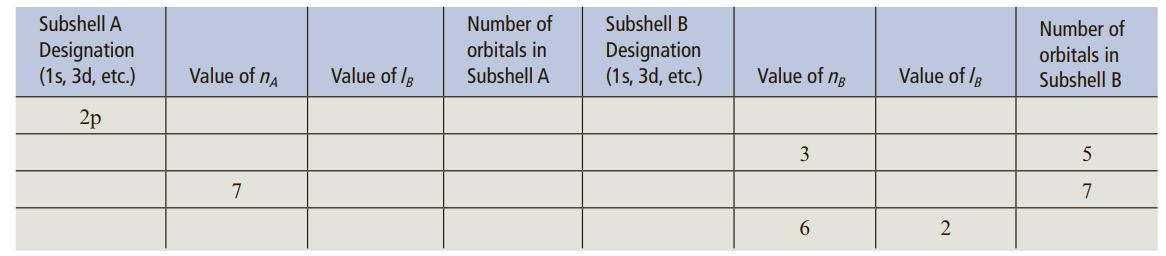
Transcribed Image Text:
Subshell A Number of Subshell B Number of Designation (1s, 3d, etc.) orbitals in Designation (1s, 3d, etc.) orbitals in Value of nA Value of /B Subshell A Value of ng Value of /B Subshell B 2p 3 7 7 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
Determining the subshells A and B is key to completing the table Once one of these is found the othe...View the full answer

Answered By

Rustia Melrod
I am a retired teacher with 6 years of experience teaching various science subjects to high school students and undergraduate students. This background enables me to be able to help tutor students who are struggling with the science of business component of their education. Teaching difficult subjects has definitely taught me patience. There is no greater joy for me than to patiently guide a student to the correct answer. When a student has that "aha!" moment, all my efforts are worth it.
The Common Core standards are a useful yardstick for measuring how well students are doing. My students consistently met or exceeded the Common Core standards for science. I believe in working with each student's individual learning styles to help them understand the material. If students were struggling with a concept, I would figure out a different way to teach or apply that concept. I was voted Teacher of the Year six times in my career. I also won an award for Innovative Teaching Style at the 2011 National Teaching Conference.
4.90+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The table below is from Exercise 4. It shows six stocks traded on either the New York Stock Exchange (NYSE) or the NASDAQ Exchange. Also shown is an indication of whether the stock gained or lost...
-
The table below is an excerpt from Apple Inc.'s Statement of f Shareholders' Equity for its fiscal year ended September 27, 2014: a. How would the auditor verify the balances as of September 28,...
-
The table below is the output of polytomous (multinomial) logistic regression of clinical classification on insulin response (IR), steady-state plasma glucose (SSPG), and relative weight (RW). The...
-
Susan recently quit working for a local firm and has yet to find a new job. She knows she can maintain her health insurance from her old employer due to COBRA. How much will it likely cost her for...
-
What is the preferred way for people to order fast food? A survey was conducted in 2009, but the sample sizes were not reported. Suppose the results, based on a sample of 100 males and 100 females,...
-
Selected accounts from Lue Co.s adjusted trial balance for the year ended December 31 follow. Prepare a classified balance sheet. Total equity .. Equipment Salaries payable Accounts receivable Cash...
-
The piece of rubber is originally rectangular. Determine the average shear strain xy at A if the corners B and D are subjected to the displacements that cause the rubber to distort as shown by the...
-
Townsend Chemical Company makes a variety of cosmetic products, one of which is a skin cream designed to reduce the signs of aging. Townsend produces a relatively small amount (15,000 units) of the...
-
(a) A wooden sphere with a diameter of d = 0.14 m (density? = 0.40 g/cm3) held under water by a string. What is the tension (in N) in the string? Note: Draw the free body diagram. (Assume the density...
-
A random number generator produces numbers between 1 and 99. If the current value of the random variable is 45, then what is the probability that the next randomly generated value for the same random...
-
The table below is partially completed for subshells A and B, where subshell B is the next subshell higher in energy compared to subshell A. For example, if subshell A is the 4s subshell, then...
-
In a hypothetical universe, the quantum numbers for an atomic orbital follow these rules: n = any positive integer value from 2 to l = any positive integer value from 2 to n + 1 m l = any integer...
-
What is believed to be the origin of atmospheric oxygen?
-
Hui is currently considering investing in municipal bonds that earn 4.65 percent interest, or in taxable bonds issued by the Coca-Cola Company that pay 6.20 percent. Required: a. If Hui's tax rate is...
-
Direct materials Direct labor $ 69,000 $ 35,000 Variable manufacturing overhead $ 15,000 Fixed manufacturing overhead 28,000 Total manufacturing overhead $ 43,000 Variable selling expense $ 12,000...
-
Millikan's oil drop experiment used to measure the elementary charge by introducing a tiny negatively charged droplet of mineral oil between the two horizontally parallel charged plates. An oil...
-
B D 2. Analyze the behavior of the above circuit with a truth table and show the table here. What is the equivalent Boolean expression?
-
1. Convert the velocity of 10 meters per second into the following types of units: Yards per hour and kilometers per week. 2. Convert the area of 1.5 square kilometers into square feet and into...
-
Do Monopolistically competitive firms generate a long-run profit? 2. Why is a monopolistic competition said to be inefficient?
-
(a) As Section 17.3 discusses, high-frequency sound waves exhibit less diffraction than low-frequency sound waves do. However, even high-frequency sound waves exhibit much more diffraction under...
-
What is the rms speed (in m/s) of a carbon tetrachloride molecule at 22oC?
-
At what temperature do hydrogen molecules, H2, have the same rms speed as nitrogen molecules, N2, at 455oC? At what temperature do hydrogen molecules have the same average kinetic energy?
-
If it takes 3.52 s for 10.0 mL of helium to effuse through a hole in a container at a particular temperature and pressure, how long would it take for 10.0 mL of oxygen, O2, to effuse from the same...
-
Discuss the dynamic organization of the cytoskeleton and its pivotal role in cellular motility, intracellular transport, and structural integrity .
-
Would you support the idea of a government issued Digital currency? Why ? and why not?
-
To protect her savings against further inflation and to help her prepare for a healthy financial future, Hanna Lind deposits $9,100 in an investment account earning 6% interest compounded quarterly....

Study smarter with the SolutionInn App


