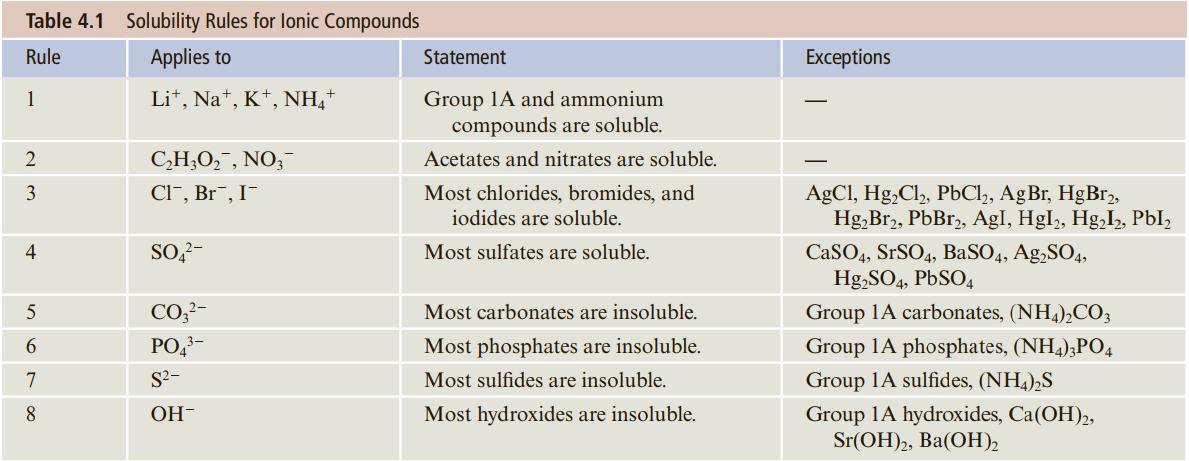
Use the solubility rules (Table 4.1) to decide which of the following compounds are expected to be
Question:
Use the solubility rules (Table 4.1) to decide which of the following compounds are expected to be soluble and which insoluble.
a. Mg(C2H3O2)2
b. NiS
c. Cr(NO3)2
d. Ca3(PO4)2
Transcribed Image Text:
Table 4.1 Solubility Rules for lonic Compounds Rule Applies to Statement Exceptions Lit, Na*, K*, NH," Group 1A and ammonium compounds are soluble. 1 C,H;O,, NO,- Acetates and nitrates are soluble. 3 CI", Br", I- Most chlorides, bromides, and AgCl, Hg,Cl,, PbCl,, AgBr, HgBr, Hg,Br2, PbBr,, AgI, Hgl, Hg,I, PbI, iodides are soluble. SO,?- CaSO4, SrSO4, BaSO4, Ag,SO4, Hg,SO4, PBSO4 4 Most sulfates are soluble. 5 CO,?- Most carbonates are insoluble. Group 1A carbonates, (NH4),CO; 6 PO,- Most phosphates are insoluble. Group 1A phosphates, (NH4),PO4 7 S2- Most sulfides are insoluble. Group 1A sulfides, (NH,),S 8 OH- Most hydroxides are insoluble. Group 1A hydroxides, Ca(OH),, Sr(ОH)2, Ba(ОН).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a MgC 2 H 3 O 2 2 is soluble acetates are solub...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the following compounds are aromatic? a. b. c. Cycloheptatrienyl cation d. e. f. g. Cyclononatetraenyl anion h. CH2=CHCH=CHCH=CH2
-
Which of the following compounds are chiral? (a) 2-Methylheptane (b) 3-Methylheptane (c) 4-Methylheptane (d) 1,1-Dibromopropane (e) 1,2-Dibromopropane (f) 1,3-Dibromopropane (g) Ethene, H2C=CH2 (h)...
-
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may...
-
Find the minimum and maximum values of the function subject to the given constraint. f(x, y) =xy, 4x +9y = 32
-
The study of whether birth decade can predict the number of software millionaires born in the decade. The data are reproduced in the table shown below. a. Construct a 95% confidence interval for the...
-
Discuss why an increasing current ratio might not be an indicator of better working capital management.
-
You are a sales manager for an office supply distributor in a large metropolitan area. What do you use as your basic control unit in creating territories? Why?
-
You are considering investing in a company that cultivates abalone for sale to local restaurants. Use the following information: Sales price per abalone ............. = $ 35 Variable costs per...
-
Freight in 16,600 Total cost of merchandise purchased Inventory available for sale Inventory, April 30, 20Y8 Cost of goods sold before estimated returns Estimated returns Cost of goods sold...
-
William is a trader. His financial year ends on 31 December. He rents business premises. The monthly rent of $1,350, payable in advance on the first day of each month, was increased to $1,380 from 1...
-
You are given a solution of the ions Mg 2+ , Ca 2+ , and Ba 2+ . Devise a scheme to separate these ions using sodium sulfate. Note that magnesium sulfate is soluble.
-
The solubility of cobalt(II) iodate in water is 1.2 g/100 mL. Calculate the solubility product constant for cobalt(II) iodate, Co(IO 3 ) 2 .
-
Goldman Company reports net income of $140,000 each year and pays an annual cash dividend of $50,000. The company holds net assets of $1,200,000 on January 1, 2010. On that date, Wallace purchases 40...
-
Compare and contrast Costco and Walmart in terms of: 1. Business Model 2. Brand positioning 3. Product, pricing 4. Place and Promotion
-
Jill has found an SUV she likes for $50,000 with no money down and 0% financing for 72 months. The dealership also offers $10,000 cash back BUT the SUV will have to be financed locally at a bank for...
-
Recall the example discussed in class where scientists utilized unsupervised machine learning to classify pictures of cancerous moles. Using the above example (or another one of your choice) explain:...
-
Imagine you are Rip Van Winkle's daughter, Judith, writing a journal entry after her father reappears after 20 years. Try to get into the mind of Judith. Be imaginative and creative. Pretend you are...
-
A bank offers to syndicate a Eurodollar loan for an MNC. The MNC has a credit risk rating of AA. Loan Type: Bullet Principal: USD 21 million Maturity: 4 years Upfront Syndication Fee: 1.95% Interest...
-
You own a U.S. exporting firm and will receive 10 million Swiss francs in one year. Assume that interest parity exists. Assume zero transactions costs. Today, the one-year interest rate in the U.S....
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Carbon monoxide and hydrogen react in the presence of a catalyst to form methanol, CH3OH: An equilibrium mixture of these three substances is suddenly compressed so that the concentrations of all...
-
A and B react to produce C according to the following chemical equation: A+ B C Amounts of A and B are added to an equilibrium reaction mixture of A, B, and C such that when equilibrium is again...
-
Given the hypothetical exothermic reaction A2(g) + 2B(g) 2AB(g) at equilibrium, decide which of the following containers represents the reaction mixture at the higher temperature? (The other...
-
Carmaker produces small cars. As production emits pollution, Carmaker needs to buy pollution permits. It also needs to hire labour. Carmaker's production function is given by: (1) q = min{0.05L,...
-
Describe the three key differences between the North American Free Trade Agreement (NAFTA) NAFTA and United States-Mexico-Canada Agreement (CUSMA). Support your answer by providing specific examples.
-
Discuss what is Brexit. Why did the United Kingdom decided to leave the European Union? What is the impact of trade between the U.K. and the E.U. post-Brexit?

Study smarter with the SolutionInn App


