Without looking at Table 9.3, arrange the following in order of increasing ionic radius: As 3 ,
Question:
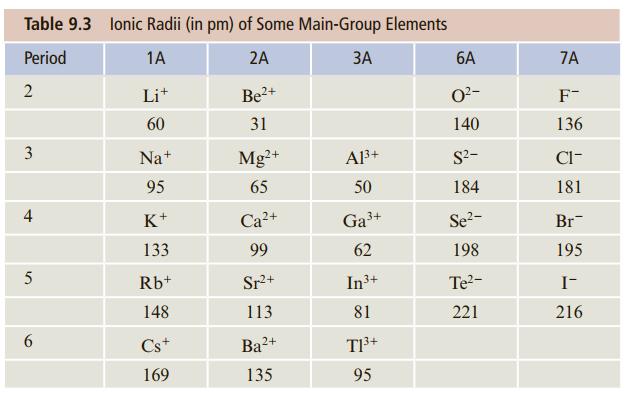
Without looking at Table 9.3, arrange the following in order of increasing ionic radius: As3−, Se2−, Br−. Explain how you arrived at this order.
Transcribed Image Text:
Table 9.3 lonic Radii (in pm) of Some Main-Group Elements Period 1A 2A ЗА 6A 7A 2 Lit 2+ O2- F- 60 31 140 136 3 Na+ Mg?+ A3+ S2- Cl- 95 65 50 184 181 K+ Ca?+ Ga3+ Se?- Br 133 99 62 198 195 5 Rb+ Sr+ In3+ Te?- I- 148 113 81 221 216 Cs+ Ba2+ 169 135 95
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Br Se 2 As 3 These ions are members of a...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following in order of increasing radius and increasing ionization energy. a. N+, N, N- b. Se, Se-, Cl, Cl+ c. Br-, Rb+, Sr2+
-
Without looking at Table 9.3, arrange the following ions in order of increasing ionic radius: Cl, Ca2+, P3. (You may use a periodic table.)
-
Arrange the following in order of increasing ionic radius: Cl, Na+, and S2. Explain this order. (You may use a periodic table.)
-
In Exercises 3336, use possible symmetry to determine whether each graph is the graph of an even function, an odd function, or a function that is neither even nor odd. ------ -2, y CI (0,4) 2 14...
-
On February 4 of a particular year, the spot rate for U.S. dollars ($) expressed in Euros () was $0.7873/. The U.S. interest rate (compounded semiannually) was 5.36 percent, whereas the euro interest...
-
What is the longest prefix of the string "cgtacgttcgtacg" that is also a suffix of this string?
-
The following table gives the vapor pressure of water for various temperatures, previously reported in Exercise 5.2. Exercise 5.2 The following table gives the vapor pressure of water for various...
-
Transactions related to revenue and cash receipts completed by Sycamore Inc. during the month of March 20Y8 are as follows: Mar. 2. Issued Invoice No. 512 to Santorini Co., $905. 4. Received cash...
-
(1) A borrower finds that the incremental cost of borrowing an extra $10,000 is 14%. If the borrower can earn 12% on alternative investments of comparable risk should he borrow the extra $10,000 at...
-
Franklin Furniture, Inc. (FFI) manufactures bedroom furniture in sets (a set includes a dresser, two queen-size beds, and one bedside table) for use in motels and hotels. FFI has three customer...
-
Arrange the following in order of increasing ionic radius: F - , Na + , and N 3- . Explain this order.
-
Arrange the members of each of the following pairs in order of increasing radius and explain the order: a. Ca, Ca 2+ b. P, P 3
-
What is the amount of depreciation, using the double-declining-balance method for the second year of use for equipment costing $9,000, with an estimated residual value of $600 and an estimated life...
-
How do leaders evaluate the effectiveness of their delegation practices, soliciting feedback, measuring performance outcomes, and iteratively refining their approach to delegation to maximize...
-
What role does interdisciplinary collaboration play in fostering visionary breakthroughs, and how can leaders facilitate cross-disciplinary exchange to spark creativity, synergize diverse...
-
2 Solve the equation x+x-6=0 by factoring. Input your solutions below in any order and round to three decimal places if necessary. x 1 = or x2 =
-
How can organizations promote resilience through targeted interventions, such as resilience training programs, organizational restructuring initiatives, and crisis management protocols, to cultivate...
-
How do lessons from decision theory and behavioral economics inform public policy interventions aimed at promoting more decisive action on pressing societal issues, such as climate change mitigation...
-
Kay, who is not a dealer, sold an apartment house to Polly during 2013. The closing statement for the sale is as follows: During 2013, Kay collected $9,000 in principal on the installment note and...
-
Solve each equation or inequality. |6x8-4 = 0
-
What are the two factors needed to explain the differences in solubilities of substances?
-
Explain how soap removes oil from a fabric.
-
Consider two hypothetical pure substances, AB(s) and XY(s). When equal molar amounts of these substances are placed in separate 500-mL samples of water, they undergo the following reactions: AB(s) ...
-
Z Find zw and z=8-8i w= 3+i Write each answer in polar form and in exponential form. The product zw in polar form is and in exponential form is (Simplify your answer. Type an exact answer, using it...
-
Consider the following equation. -2d+16=2(d+8) - 4d Solve the equation. d The solution set is 0. The solution set is {d | d is a real number}. Classify the equation as either an identity, consistent...
-
What innovative approaches can organizations employ to reconcile competing demands for capital investment and operational expenditure within the constraints of finite budgetary resources, while still...

Study smarter with the SolutionInn App


