A researcher is using a 5-cm-diameter Stefan tube to measure the mass diffusivity of chloroform in air
Question:
A researcher is using a 5-cm-diameter Stefan tube to measure the mass diffusivity of chloroform in air at 25°C and 1 atm. Initially, the liquid chloroform surface was 7.00 cm from the top of the tube; and after 10 hours have elapsed, the liquid chloroform surface was 7.44 cm from the top of the tube, which corresponds to 222 g of chloroform being diffused. At 25°C, the chloroform vapor pressure is 0.263 atm, and the concentration of chloroform is zero at the top of the tube. If the molar mass of chloroform is 119.39 kg/kmol, determine the mass diffusivity of chloroform in air.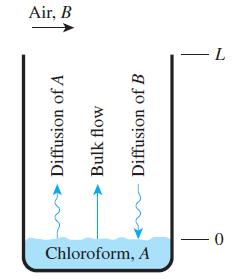
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Heat And Mass Transfer Fundamentals And Applications
ISBN: 9780073398181
5th Edition
Authors: Yunus Cengel, Afshin Ghajar
Question Posted:





