A water solution containing 0.005 mole fraction of benzoic acid is to be extracted using pure benzene
Question:
A water solution containing 0.005 mole fraction of benzoic acid is to be extracted using pure benzene as the solvent. If the feed rate is \(100 \mathrm{~mol} / \mathrm{h}\) and the solvent rate is \(10 \mathrm{~mol} / \mathrm{h}\), find the number of equilibrium stages required to reduce the concentration of benzoic acid in the aqueous solution to \(0.0001 \mathrm{~mol}\) fraction. Operation is isothermal at \(280 \mathrm{~K}\) (water and benzene are insoluble at this temperature), where the equilibrium data can be represented as (Wankat, 1988)
\(\mathrm{mol}\) fraction of benzoic acid in water \(=0.0446 \times(\mathrm{mol}\) fraction of benzoic acid in benzene)
Problems 7.9 to 7.12 refer to the system water (A)-chlorobenzene (B)-pyridine (C) at 298 K. Equilibrium tie-line data taken from Treybal (1980) in weight percent are given in Table 7.7.
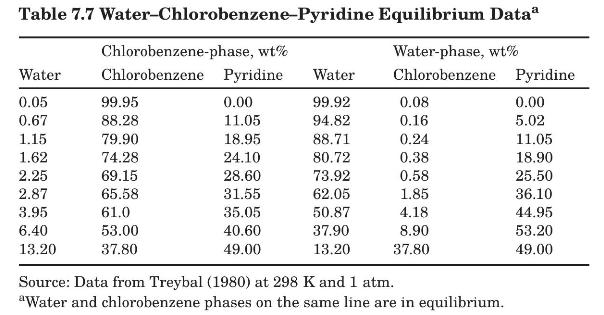
Data From Problem 7.9 to 7.12:-
Problem 7.9:-
Plot the equilibrium data for the system water-chlorobenzene-pyridine in right-triangular coordinates.
Problem 7.10:-
It is desired to reduce the pyridine concentration of \(5000 \mathrm{~kg} / \mathrm{h}\) of an aqueous solution from \(50 \mathrm{wt} \%\) to \(5 \mathrm{wt} \%\) in a single batch extraction with pure chlorobenzene. What amount of solvent is required? Solve on right-triangular coordinates. Corroborate your results using the Mathcad or Python programs of Appendix G-1 and G-2.
Data From Appendix G-1:-

Data From Appendix G-2:-

Problem 7.11:-
A \(5000-\mathrm{kg}\) batch of pyridine-water solution, \(50 \mathrm{wt} \%\) pyridine, is to be extracted with an equal weight of pure chlorobenzene. The raffinate from the first extraction is to be reextracted with a weight of pure solvent equal to the raffinate weight, and so on ( \(S_{2}=R_{1}, S_{3}=R_{2}\), etc.). How many ideal stages and what total solvent are required to reduce the concentration of pyridine to no more than \(2 \mathrm{wt} \%\) in the final raffinate?
Problem 7.12:-
A pyridine-water solution, \(50 \mathrm{wt} \%\) pyridine, is to be continuously and countercurrently extracted at the rate of \(2.25 \mathrm{~kg} / \mathrm{s}\) with pure chlorobenzene to reduce the pyridine concentration to \(2 \mathrm{wt} \%\).
(a) Determine the minimum solvent rate required.
(b) If \(2.3 \mathrm{~kg} / \mathrm{s}\) of solvent is used, calculate the number of theoretical stages required, and the flow rates of final extract and raffinate.
Step by Step Answer:





